Piperlonguminine
Piperlonguminine (CAS 5950-12-9) is a bioactive compound isolated from Piper longum that exhibits anticancer, antihyperlipidemic, and anti-inflammatory activities. Mechanistically, piperlonguminine reduces melanin synthesis in melanoma B16 cells by suppressing α-MSH-induced signaling through cyclic AMP, thereby decreasing tyrosinase expression. Additionally, it demonstrates neuroprotective effects in rat models of cerebral ischemia by reducing NF-κB and MAPK activation, resulting in decreased neurological deficits, infarct size, and cerebral edema. Piperlonguminine is currently under preclinical investigation for cancer, neurological disorders, and inflammation.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 273.3 |
| Cas No. | 5950-12-9 |
| Formula | C16H19NO3 |
| Synonyms | N-Isobutylpiperamide|NSC 125178 |
| Solubility | ≥25.9 mg/mL in DMSO; ≥21.3 mg/mL in EtOH; insoluble in H2O |
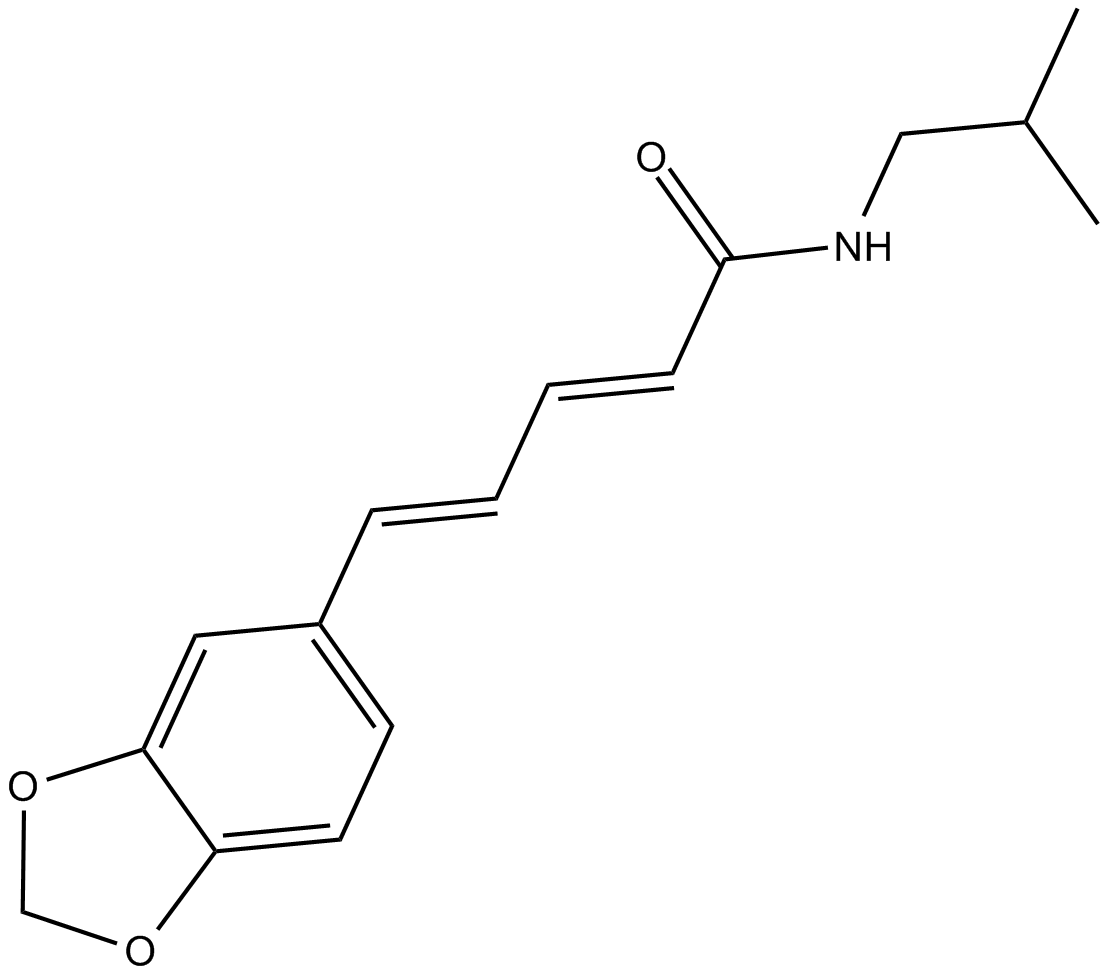
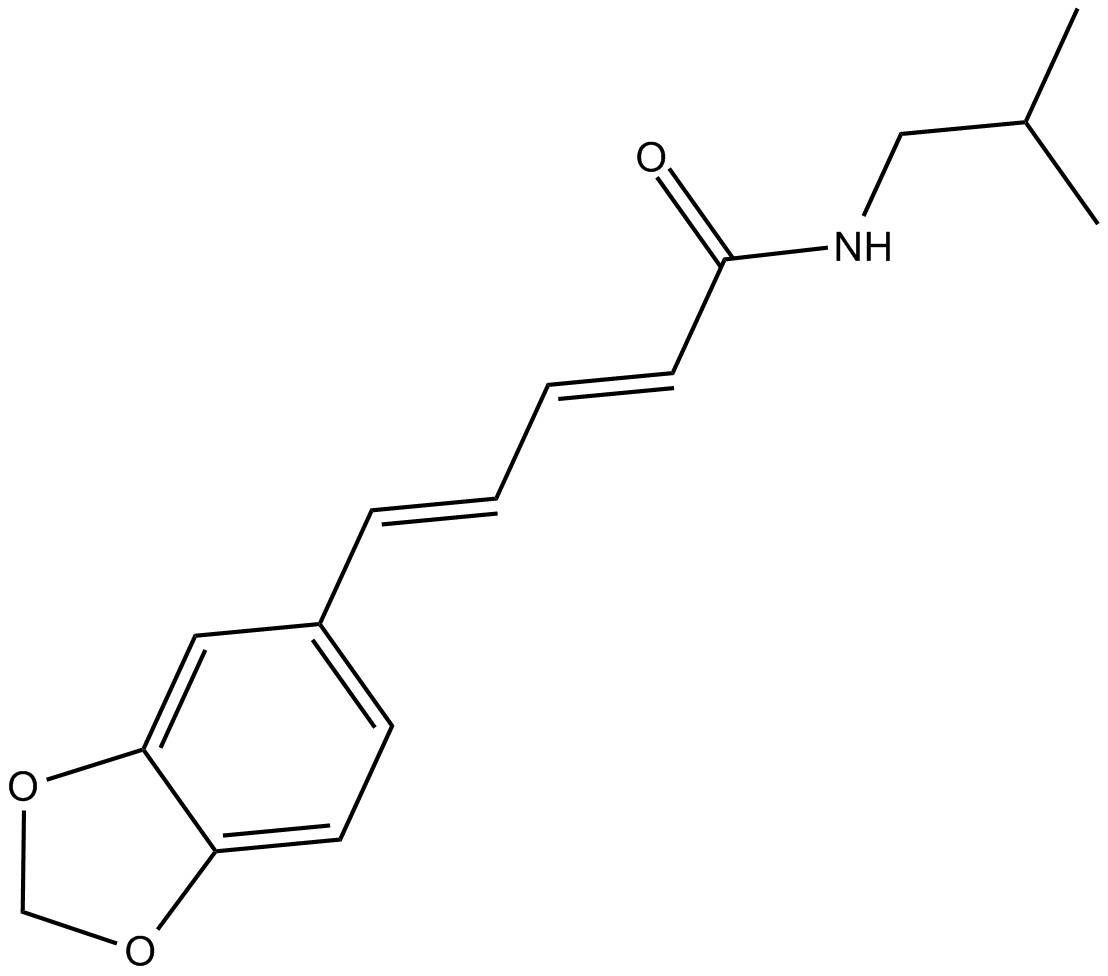
| Chemical Name | 5-(1,3-benzodioxol-5-yl)-N-(2-methylpropyl)-2E,4E-pentadienamide |
| SDF | Download SDF |
| Canonical SMILES | CC(C)CNC(/C=C/C=C/c(cc1)cc2c1OCO2)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Melanoma B16 cells |
|
Preparation method |
The solubility of this compound in DMSO is ≤ 20mg/ml. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
3~30 μM |
|
Applications |
Piperlonguminine does not alter 1-oleoyl-2-acetyl-sn-glycerin-induced melanin production, nor does it affect protein kinase C-mediated melanin production. In addition, piperlonguminine cannot inhibit the catalytic activity of cell-free tyrosinase in melanoma B16 cells, which is attributed to the inhibitory effect of piperlonguminine on the α-MSH-induced signal of cAMP to cAMP response element binding protein. |
| Animal experiment [1]: | |
|
Animal models |
Cerebral ischemia rats |
|
Dosage form |
2.4 mg/kg (i.p.) |
|
Application |
Intraperitoneal injection of piperlonguminine (2.4 mg/kg) has obvious neuroprotective effect on cerebral ischemia rats. Piperlonguminine attenuates neurological deficit scores, cerebral infarct volume and brain water content in rats, and inhibits the activation of NF-κB and MAPK. These data suggest that piperlonguminine protects the brain from ischemic brain damage by reducing the damage to the blood-brain barrier (BBB), which may be mediated by inhibiting NF-κB and MAPK signaling pathways. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Kim KS, Kim JA, Eom SY, Lee SH, Min KR, Kim Y. Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression. Pigment Cell Res. 2006 Feb;19(1):90-8. PubMed PMID: 16420250. [2]. Yang T, Sun S, Wang T, Tong X, Bi J, Wang Y, Sun Z. Piperlonguminine is neuroprotective in experimental rat stroke. Int Immunopharmacol. 2014 Dec;23(2):447-51. doi: 10.1016/j.intimp.2014.09.016. Epub 2014 Sep 22. PubMed PMID: 25257731. |
|
Quality Control & MSDS
- View current batch:
Chemical structure