PCR Mycoplasma Detection Kit
PCR Mycoplasma Detection Kit provides specialized nested PCR-based detection of mycoplasma contamination in cell cultures frequently used in biomedical research laboratories. Mycoplasma, as small prokaryotes (0.1–0.3 μm), represent common contaminants capable of widespread cellular disruptions and genetic alterations, thus periodic screening is essential in routine cell line cultivation. This assay applies nested PCR technology, employing two sequential amplification reactions targeting conserved 16S and 23S rRNA coding regions and the intergenic spacer DNA region of Mycoplasma, ensuring higher detection sensitivity and specificity than conventional PCR. Inclusion of positive controls allows monitoring of PCR reactions and detection of PCR inhibitors from test samples. Detected contaminated cells may be eliminated or treated appropriately. This kit supports research requiring verified mycoplasma-free cells, including transfections (efficiency ≥80% across HEK293, HeLa), antibiotic-resistant bacterial screenings (100 μg/ml ampicillin solutions), competent cell preparations (E.coli DH5α genotype: fhuA2 Δ(argF-lacZ)U169 recA1 endA1 hsdr17 supE44 thi-1 gyrA96 relA1; typical transformation efficiency ≥10^8 cfu/μg DNA), and cell cryopreservation (recovery rate ≥90%).
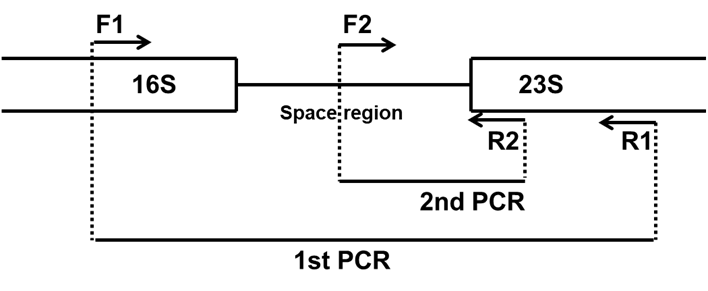
Figure 1: Principle of nested PCR-based mycoplasma detection
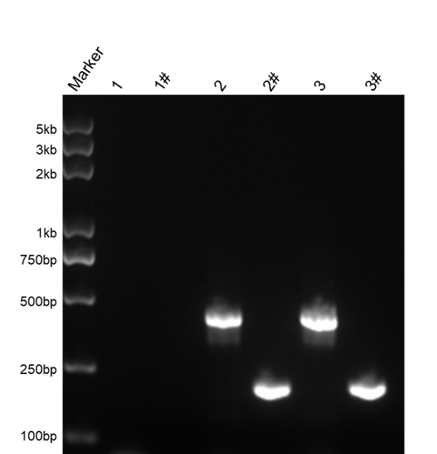
Figure 2: Schematic of agarose gel electrophoresis of 1st PCR and 2nd PCR products.
1, 2, 3 are 1st PCR products; 1#, 2#, 3# are the 2nd PCR products. The templates for each lane are: 1 and 1#, negative cell supernatant; 2 and 2#, mycoplasma contaminated cell supernatant; 3 and 3#, Positive Control.
| Components | K2821-50 T | K2821-100 T |
| 2X Taq PCR Master Mix (with dye) | 1 mL | 2 x 1 mL |
| 1st PCR Primer Mix (50X) | 20 µL | 40 µL |
| 2nd PCR Primer Mix (50X) | 20 µL | 40 µL |
| Positive Control | 20 µL | 40 µL |
Store the kit at -20°C, avoiding repeated freeze and thaw cycles, stable for 1 year. | ||








