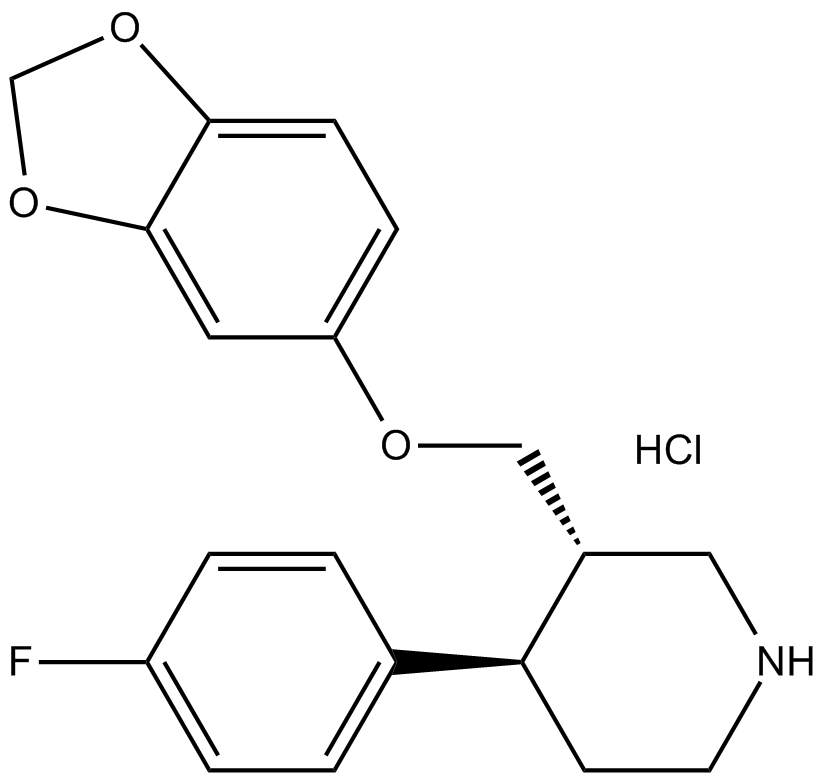
Paroxetine HCl
Paroxetine Hydrochloride is a potent and selective serotonin reuptake inhibitor (SSRI) . This compound exerts antidepressant effects by specifically binding to the presynaptic serotonin transporter (SERT), thereby elevating synaptic 5-HT concentrations (IC₅₀ = 14 μM). Recent studies reveal its novel capacity to inhibit G protein-coupled receptor kinase 2 (GRK2) phosphorylation activity, suggesting potential applications in neurosignal transduction modulation.
As an established active pharmaceutical ingredient (API), paroxetine hydrochloride is extensively utilized in mechanistic studies and drug development for major depressive disorder (MDD), obsessive-compulsive disorder (OCD), and panic disorder. Its distinctive spatial configuration, featuring a piperidine ring fused to a benzodioxol moiety, ensures high-affinity target engagement. The product is ideal for:
- In vitro neurotransmitter metabolism studies
- CNS drug screening platforms
- GPCR signaling pathway exploration
- 1. Liang Yang, Chen Guo, et al. "Stress dynamically modulates neuronal autophagy to gate depression onset." Nature. 2025 May;641(8065):E14. PMID: 40205038
- 2. Emily M Walker, Gemma L Pearson, et al. "Retrograde mitochondrial signaling governs the identity and maturity of metabolic tissues." Science. 2025 Apr11;388(6743):eadf2034. PMID: 39913641
- 3. Xiaohang Zheng, Jianxin Qiu, et al. "Paroxetine Attenuates Chondrocyte Pyroptosis and Inhibits Osteoclast Formation by Inhibiting NF-κB Pathway Activation to Delay Osteoarthritis Progression." Drug Des Devel Ther. 2023 Aug 16;17:2383-2399. PMID: 37605762
- 4. Helen M Collins, Raquel Pinacho, et al. "Effect of selective serotonin reuptake inhibitor discontinuation on anxiety-like behaviours in mice." J Psychopharmacol. 2022 Jul;36(7):794-805. PMID: 35607713
- 5. Jang WJ, Jung SK, et al. "Anticancer activity of paroxetine in human colon cancer cells: Involvement of MET and ERBB3." J Cell Mol Med. 2018 Nov 13. PMID: 30421568
| Storage | Store at -20°C |
| M.Wt | 365.83 |
| Cas No. | 78246-49-8 |
| Formula | C19H20FNO3·HCl |
| Synonyms | BRL29060 hydrochloride; BRL29060A |
| Solubility | insoluble in H2O; ≥17.8 mg/mL in EtOH; ≥36.73 mg/mL in DMSO |
| Chemical Name | (3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine;hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | Fc1ccc([C@H]2[C@H](COc(cc3)cc4c3OCO4)CNCC2)cc1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[1] | |
|
Cell lines |
Splenic T cells |
|
Reaction Conditions |
0.1, 1 and 10 μM paroxetine |
|
Applications |
Paroxetine (1 μM and 10 μM) pretreatment distinctly restrained T cell migration induced by CX3CL1 through inhibiting G protein-coupled receptor kinase 2 (GRK2). Paroxetine also inhibited GRK2 induced activation of ERK. Thus, paroxetine could potentially attenuate the symptoms of collagen-induced arthritis due to its inhibitory effect on T cell activation and infiltration to synovial tissue via suppression of ERK pathway. |
| Animal experiment:[2] | |
|
Animal models |
Male CFY rats, 125 ~ 250 g |
|
Dosage form |
0.3, 1, 3 and 6 mg/kg Oral administration |
|
Applications |
Paroxetine HCl dose-dependently prevented the 5-HT depleting effect of p-chloroamphetamine (PCA) in rat brain, demonstrating 5-HT uptake blockade in vivo. At the highest dose tested (6 mg/kg), paroxetine HCl completely prevented the PCA effect. Paroxetine HCl (0.3 and 6.0 mg/kg), administered in the absence of PCA, produced significant increases in brain 5-HT concentrations. Therefore, paroxetine HCl could provide a useful pharmacological tool for investigating 5-HT systems. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Wang Q, Wang L, Wu L, et al. Paroxetine alleviates T lymphocyte activation and infiltration to joints of collagen-induced arthritis. Scientific Reports, 2017, 7: 45364. 2. Thomas DR, Nelson DR, Johnson AM. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology (Berl), 1987, 93(2): 193-200. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data