Nifuroxazide
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 275.22 |
| Cas No. | 965-52-6 |
| Formula | C12H9N3O5 |
| Solubility | ≥27.5 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
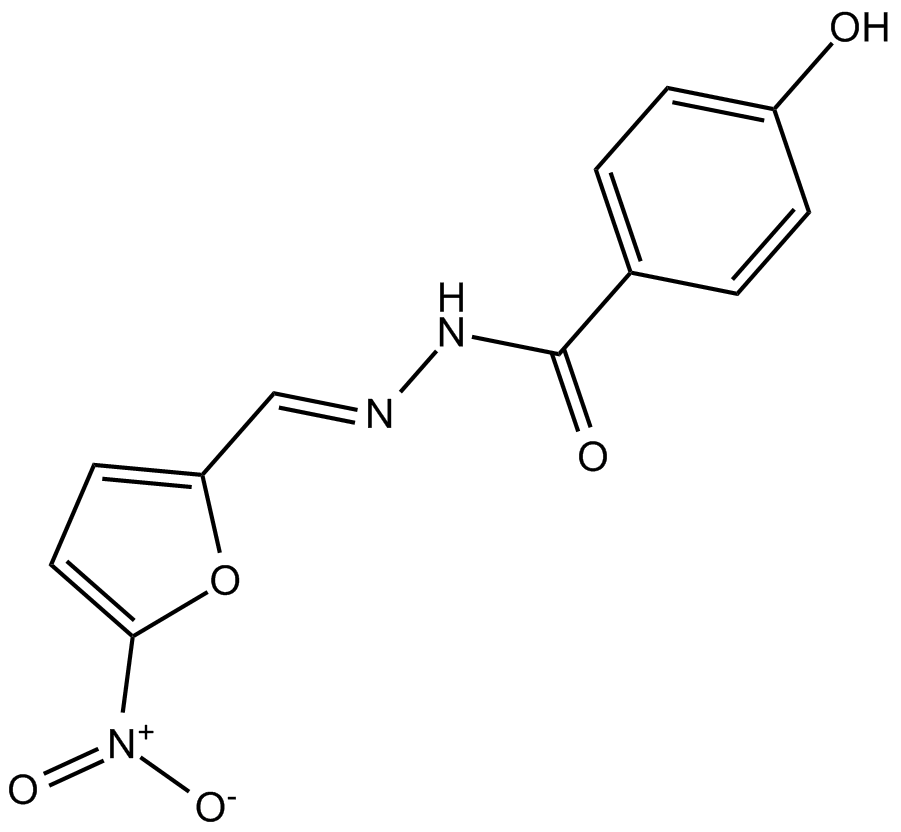
| Chemical Name | 4-hydroxy-N-[(E)-(5-nitrofuran-2-yl)methylideneamino]benzamide |
| SDF | Download SDF |
| Canonical SMILES | [O-][N+](c1ccc(C=NNC(c(cc2)ccc2O)=O)[o]1)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
U266 myeloma cells |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months. |
|
Reacting condition |
0 ~ 10 μM; 0 ~ 24 hrs |
|
Applications |
In U266 myeloma cells, Nifuroxazide significantly inhibited STAT3 tyrosine phosphorylation in a dose-dependent manner, with an IC50 value of 10 μM. The inhibitory effect of Nifuroxazide on STAT3 tyrosine phosphorylation was rapid, occurring as early as 1 hr after treatment and sustained for at least 24 hrs. In addition, Nifuroxazide did not affect STAT3 serine (727) phosphorylation. |
| Animal experiment [2]: | |
|
Animal models |
4T1 tumor-bearing mice |
|
Dosage form |
10 or 50 mg/kg; i.p.; q.d., for 24 days |
|
Applications |
In 4T1 tumor-bearing mice, Nifuroxazide significantly reduced tumor outgrowth and tumor weight, without causing any significant change in body weight. The immunohistochemical staining results of 4T1 tumors collected on day 31 showed that Nifuroxazide substantially reduced Ki-67-positive cells and increased CC-3-positive cells, indicating that Nifuroxazide inhibited cell proliferation and induced apoptosis in breast tumor tissues by inhibiting Ki-67 and activating caspase-3. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Nelson EA, Walker SR, Kepich A, Gashin LB, Hideshima T, Ikeda H, Chauhan D, Anderson KC, Frank DA. Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood. 2008 Dec 15;112(13):5095-102. [2]. Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X, Li Y, Jie H, Liu C, Xiong Y, Zuo Z, Zeng A, Li Y, Yu L, Shen G, Wang D, Xie Y, Ye T, Wei Y. Nifuroxazide induces apoptosis and impairs pulmonary metastasis in breast cancer model. Cell Death Dis. 2015 Mar 26;6:e1701. | |
Quality Control & MSDS
- View current batch:
Chemical structure