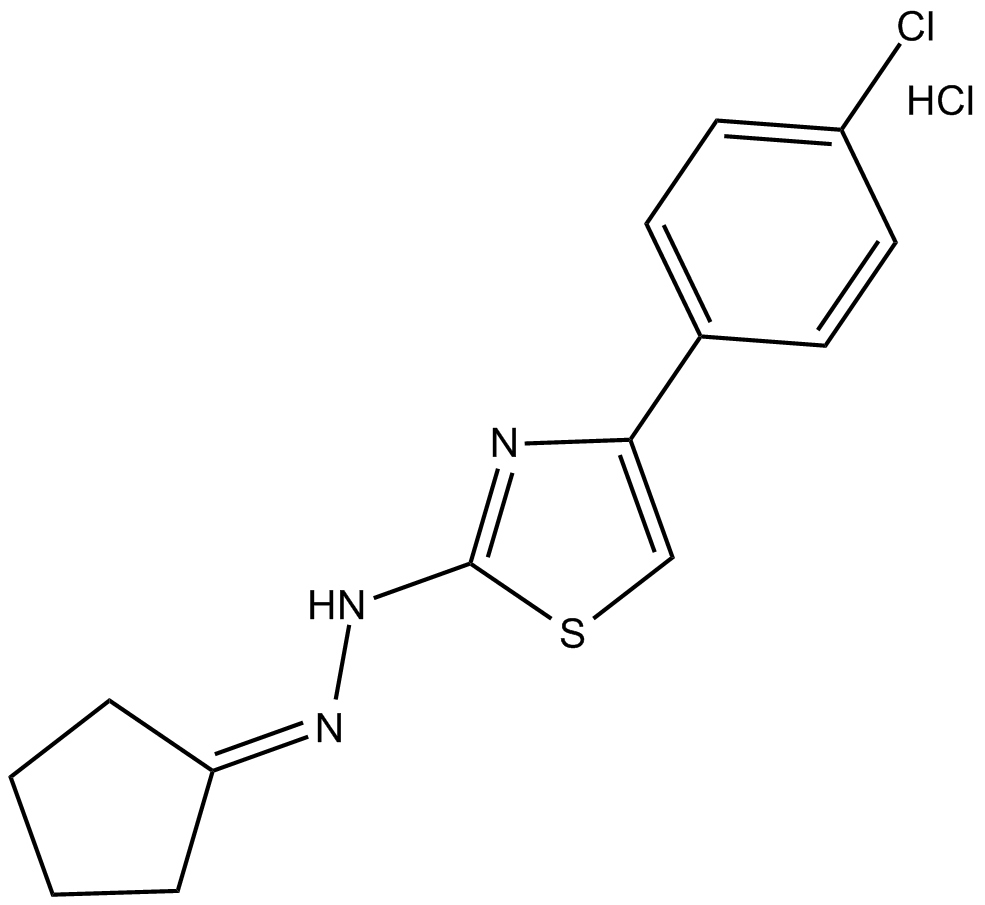
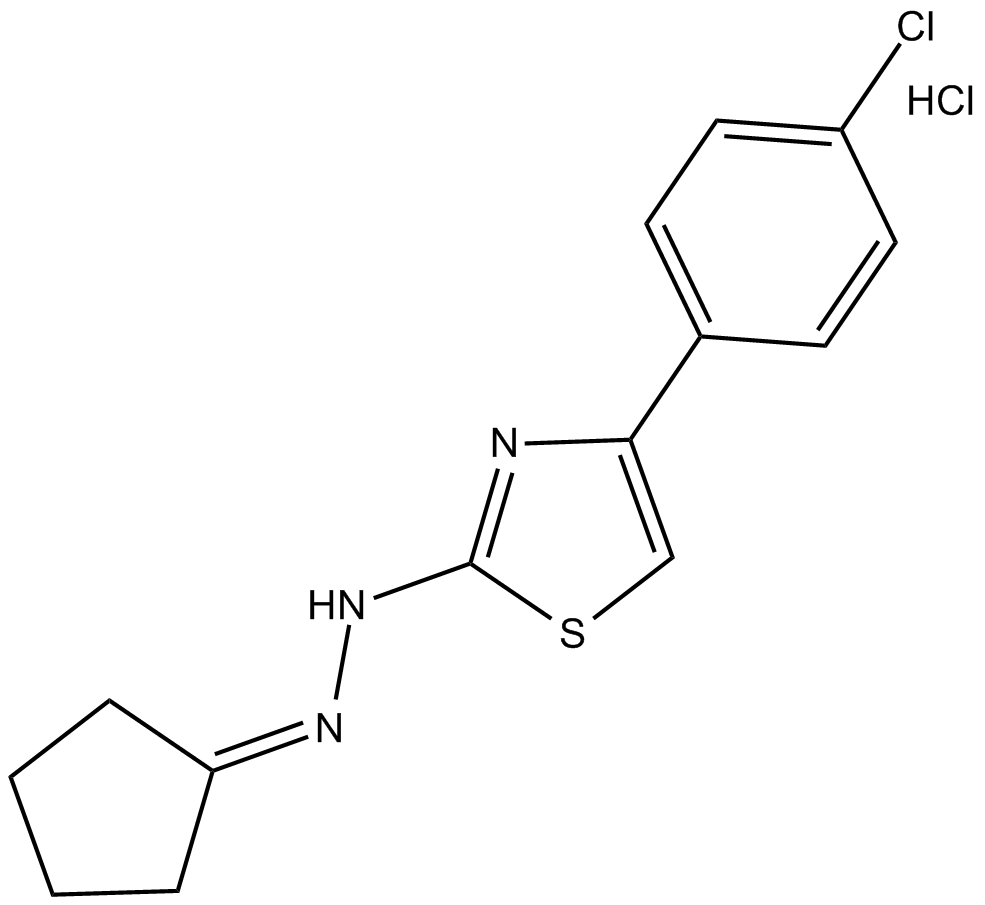
CPTH2 (hydrochloride)
CPTH2 is an inhibitor of HAT activity of Gcn5.
Histone acetyltransferases (HATs) has been identified to add the acetyl group on the specific lysine of histone H3 and H4 N-termini, and such signatures are able to increase the accessibility of the underlying chromatin at specific genes or over vast regions of the genome. Gcn5p is a chimeric protein made up of a number of functional domains.
In vitro: Previous study identified a novel molecule named CPTH2, which was selected based on its inhibitory effect on the growth of a gcn5Delta strain. This study indicated a specific chemical-genetic interaction between CPTH2 and HAT Gcn5p, suggesting that CPTH2 could inhibit the dependent functional network of Gcn5p. In addition, CPTH2 was found to be able to inhibit an in-vitro HAT reaction, which could be reverted by increasing concentration of histone H3 [1].
In vivo: In vivo, CPTH2 could decrease the acetylation of bulk histone H3 at the specific H3-AcK14 site [1].
Clinical trial: Up to now, CPTH2 is still in the preclinical development stage.
Reference:
[1] F. Chimenti, B. Bizzarri, E. Maccioni, et al. A novel histone acetyltransferase inhibitor modulating Gcn5 network: Cyclopentylidene-[4-(4'-chlorophenyl)thiazol-2-yl)hydrazone. Journal of Medicinal Chemistry 52, 530-536 (2009).
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 328.3 |
| Formula | C14H14ClN3S·HCl |
| Solubility | ≤5mg/ml in ethanol;16mg/ml in DMSO;5mg/ml in dimethyl formamide |
| Chemical Name | 2-[4-(4-chlorophenyl)-2-thiazolyl]hydrazone-cyclopentanone, monohydrochloride |
| SDF | Download SDF |
| Canonical SMILES | Clc(cc1)ccc1-c1c[s]c(NN=C2CCCC2)n1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure