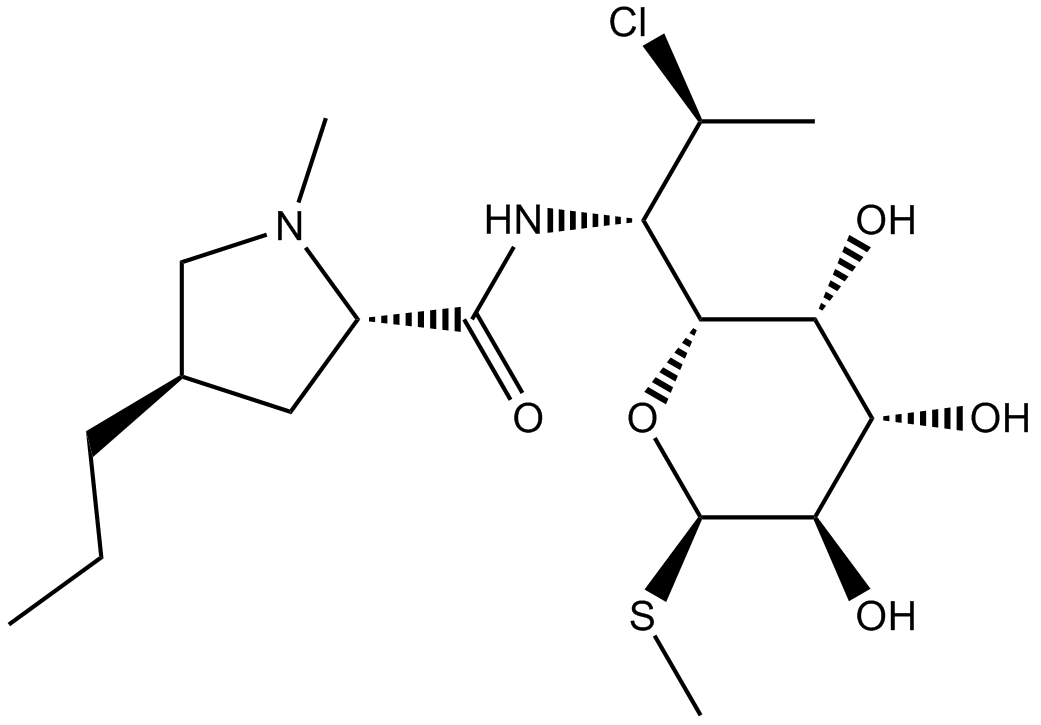
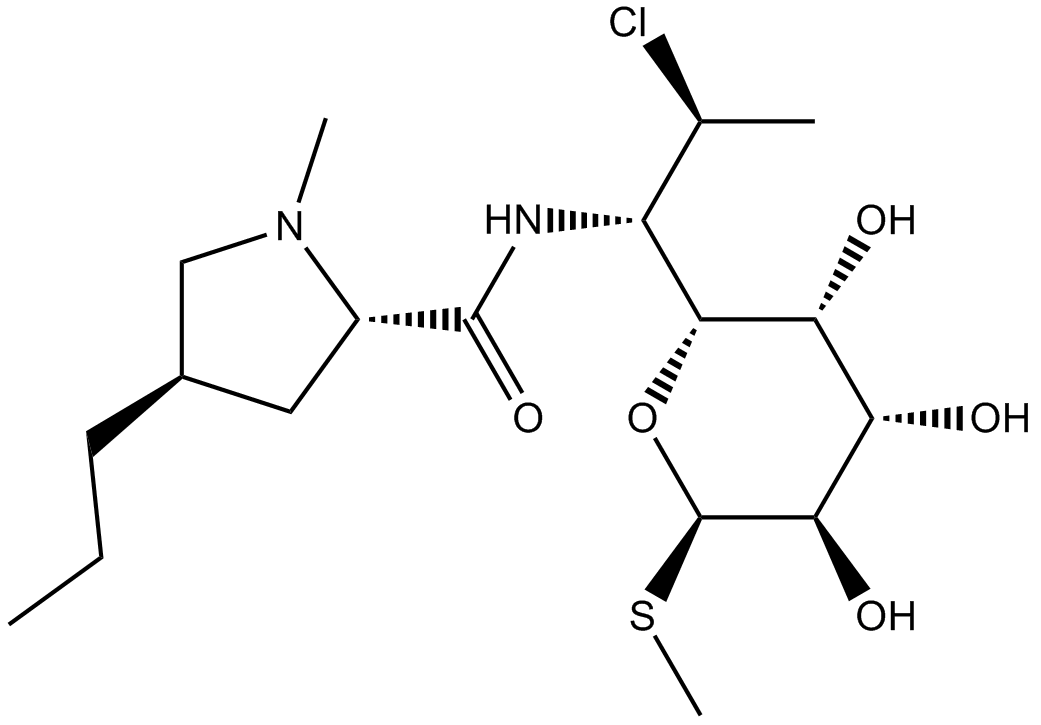
Clindamycin
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 424.98 |
| Cas No. | 18323-44-9 |
| Formula | C18H33ClN2O5S |
| Solubility | insoluble in EtOH; ≥15.3 mg/mL in DMSO; ≥50.6 mg/mL in H2O |
| Chemical Name | (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | CCC[C@H](C1)CN(C)[C@@H]1C(N[C@H]([C@H](C)Cl)[C@H]([C@@H]([C@@H]([C@H]1O)O)O)O[C@@H]1SC)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Animal experiment:[1] | |
|
Animal models |
BALB/cJ mice infected with influenza virus and superinfected with Streptococcus pneumonia (S. pneumoniae) |
|
Dosage form |
60 mg/kg Twice daily by intraperitoneal route for 7 days |
|
Applications |
In the model, clindamycin therapy used either alone (survival rate, 82%) or in combination with ampicillin (80%) performed significantly better than ampicillin therapy (56%). Improved survival appeared to be mediated by decreased inflammation manifested as lower levels of inflammatory cells and proinflammatory cytokines in the lungs and by observation of less-severe histopathologic findings. Thus, treatment with protein synthesis inhibitor clindamycin could improve outcomes of secondary bacterial pneumonia after influenza. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Karlström A, Boyd KL, English BK, et al. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. The Journal of Infectious Diseases, 2009, 199(3): 311-319. |
|
Quality Control & MSDS
- View current batch:
Chemical structure