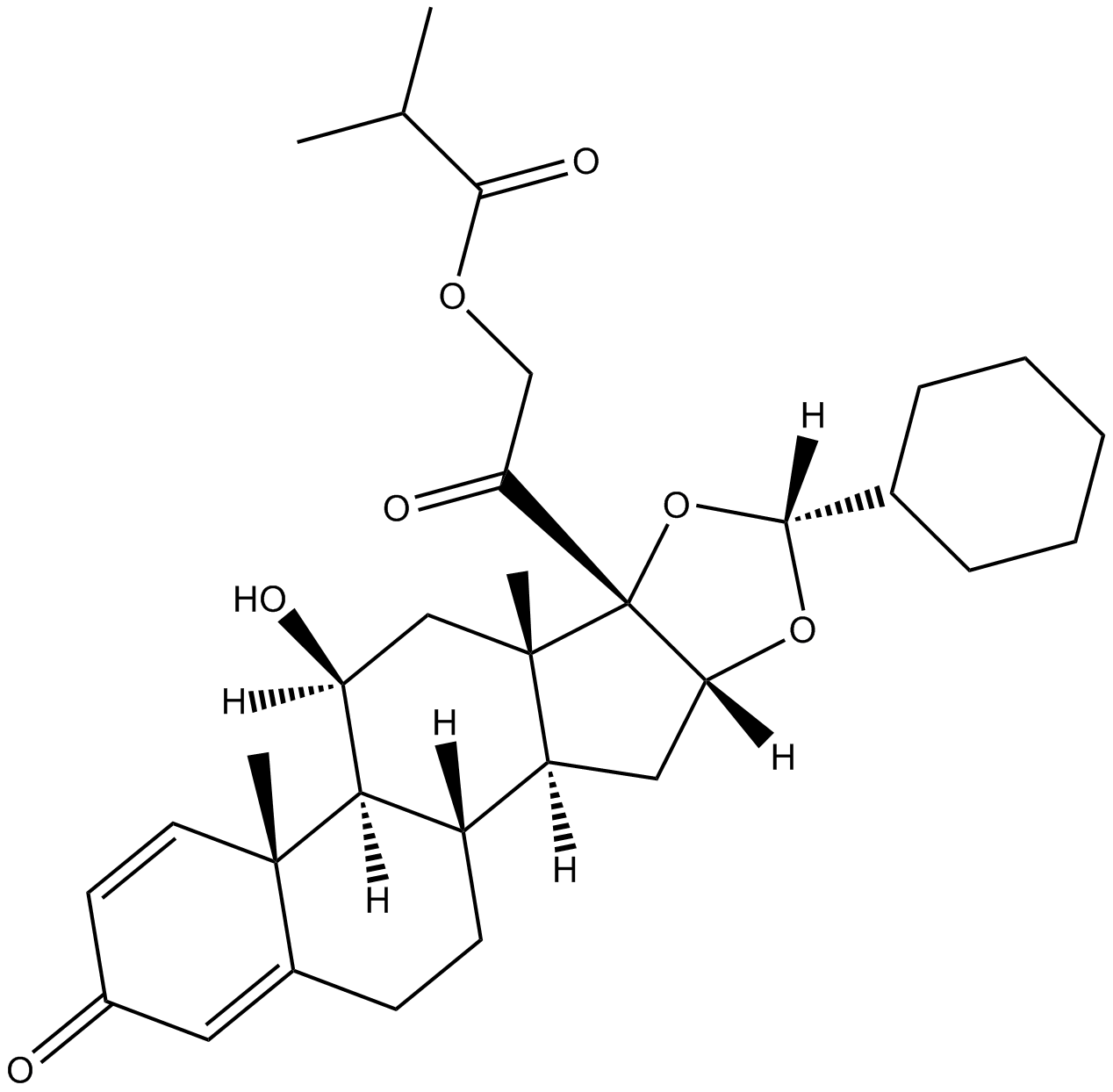
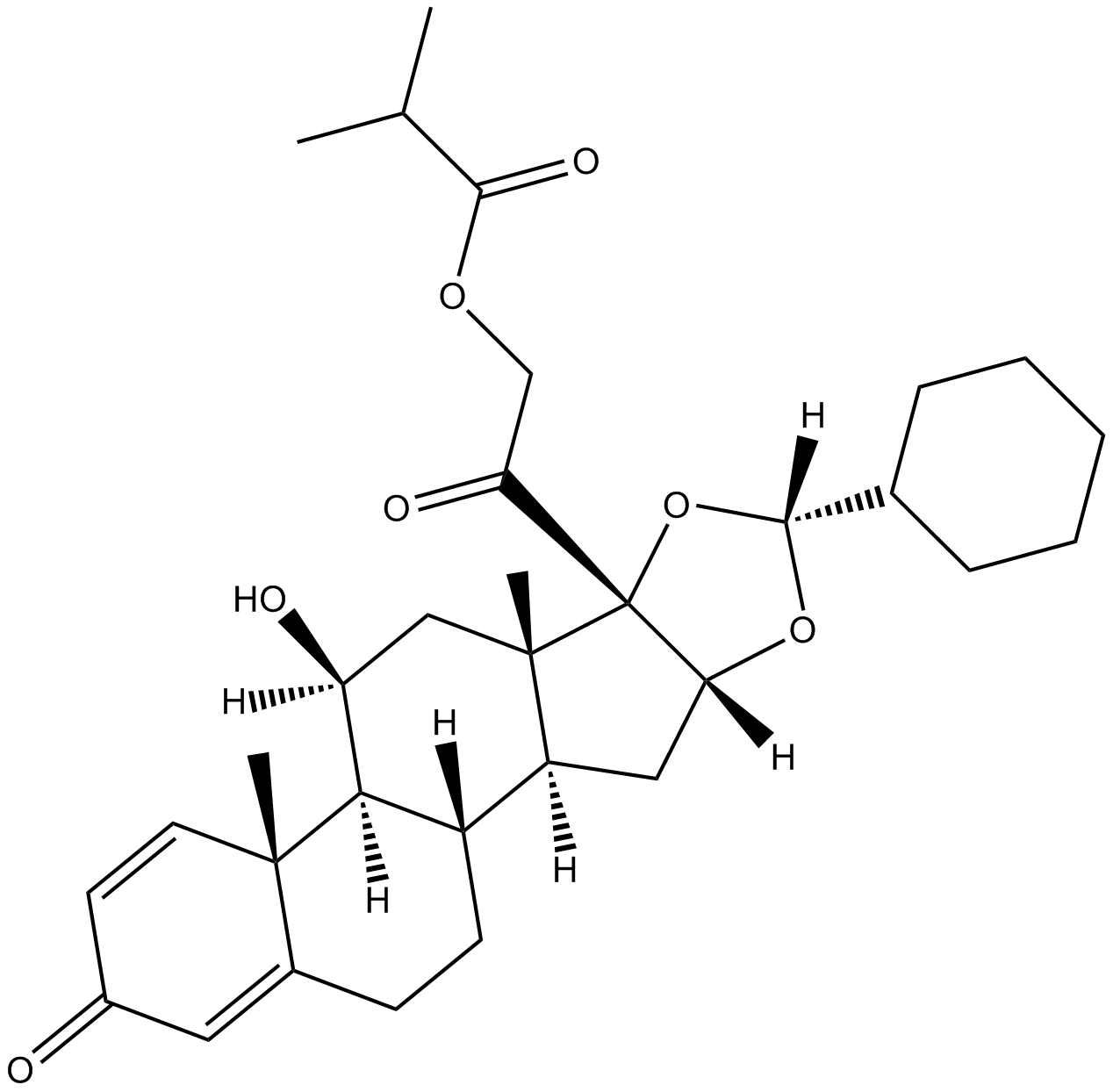
Ciclesonide
Ciclesonide is a prodrug of the potent glucocorticoid receptor agonist desisobutyryl-ciclesonide. Ciclesonide undergoes the hydrolysis process by ester cleavage at the C21 position to desisobutyryl-ciclesonide, followed by reversible formation of fatty acid esters in lung cells. Although both ciclesonide and desisobutyryl-ciclesonide are able to bind to the glucocorticoid receptor, the active metabolite desisobutyryl-ciclesonide (IC50 = 210 nM) is about 100-fold more potent than the parent compound ciclesonide (IC50 = 1.75 nM). Formulations containing ciclesonide have been used in the maintenance treatment of asthma and in the treatment of allergic rhinitis.
References:
1. Derendorf H, Nave R, Drollmann A, et al. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. European Respiratory Journal, 2006, 28(5): 1042-1050.
2. Mutch E, Nave R, McCracken N, et al. The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue. Biochemical Pharmacology, 2007, 73(10): 1657-1664.
3. Belvisi MG, Bundschuh DS, Stoeck M, et al. Preclinical profile of ciclesonide, a novel corticosteroid for the treatment of asthma. Journal of Pharmacology and Experimental Therapeutics, 2005, 314(2): 568-574.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 540.69 |
| Cas No. | 126544-47-6 |
| Formula | C32H44O7 |
| Solubility | insoluble in H2O; ≥15.8 mg/mL in DMSO; ≥50.6 mg/mL in EtOH |
| Chemical Name | 2-((6aR,6bS,7S,8aS,8bS,10R,11aR,12aS,12bS)-10-cyclohexyl-7-hydroxy-6a,8a-dimethyl-4-oxo-2,4,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-1H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-8b-yl)-2-oxoethyl isobutyrate |
| SDF | Download SDF |
| Canonical SMILES | O=C([C@]([C@@]1([H])C[C@@]2([H])[C@@](CCC3=CC4=O)([H])[C@]([C@]3(C=C4)C)([H])[C@@H](O)C5)(O[C@H](C6CCCCC6)O1)[C@]25C)COC(C(C)C)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment:[2] | |
|
Cell lines |
Normal human bronchial epithelial (NHBE) cells |
|
Reaction Conditions |
5 μM ciclesonide for 24 h incubation |
|
Applications |
Ciclesonide (5 μM) was rapidly hydrolyzed to desisobutyryl-ciclesonide by NHBE cells (approximately 30% conversion within the first 4 h), and 96% conversion of ciclesonide to desisobutyryl-ciclesonide was achieved at 24 h. |
| Animal experiment:[3] | |
|
Animal models |
Ovalbumin-sensitized and challenged Brown Norway rats |
|
Dosage form |
0 ~ 10 mg/kg Administered by the intratracheal route into the airways 24 and 1 h before exposure to inhaled antigen |
|
Applications |
Ciclesonide dose-dependently suppressed the antigen-induced influx of eosinophils into the airway lumen, with an ED50 value of 0.75 mg/kg. Moreover, ciclesonide significantly inhibited eosinophil influx into the lung tissue, with an ED50 value of 0.49 mg/kg. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Derendorf H, Nave R, Drollmann A, et al. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. European Respiratory Journal, 2006, 28(5): 1042-1050. 2. Mutch E, Nave R, McCracken N, et al. The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue. Biochemical Pharmacology, 2007, 73(10): 1657-1664. 3. Belvisi MG, Bundschuh DS, Stoeck M, et al. Preclinical profile of ciclesonide, a novel corticosteroid for the treatment of asthma. Journal of Pharmacology and Experimental Therapeutics, 2005, 314(2): 568-574. |
|
Quality Control & MSDS
- View current batch:
Chemical structure