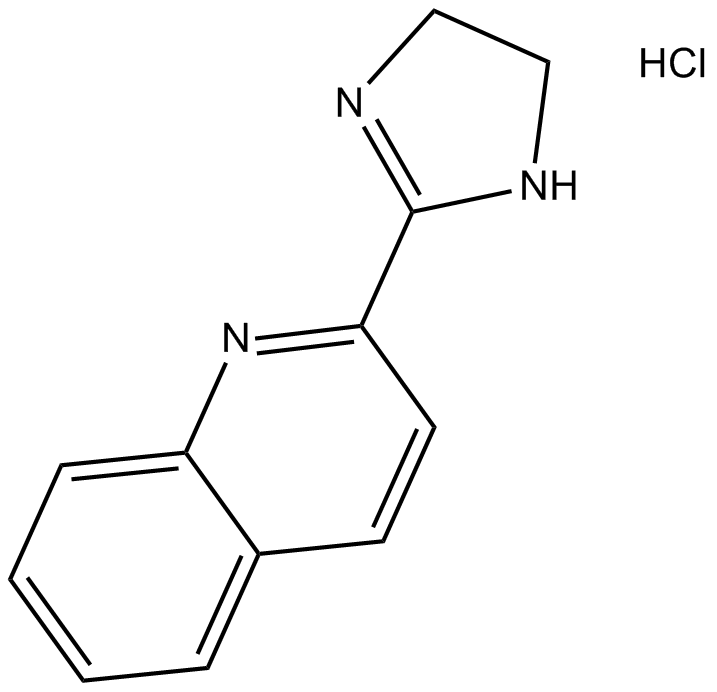
BU 224 hydrochloride
BU 224 hydrochloride is a selective ligand of the imidazoline I2 receptor with Ki value of 2.1 nM [1].
Imidazoline receptor is the primary receptor for clonidine and other imidazolines. Imidazoline I2 receptor is an allosteric binding site of monoamine oxidase and plays a critical role in neuroprotection and pain modulation.
BU 224 hydrochloride is a selective ligand of the imidazoline I2 receptor. In the rat forced swim test (FST), BU224 significantly inhibited immobility and increased mild swimming. Also, BU224 increased adrenocorticotrophic hormone (ACTH) response to FST and 5-hydroxytryptamine (5-HT) levels in frontal cortex and reduced 5-HT turnover in the hypothalamus and frontal cortex. These results showed the antidepressant-like activity of BU224 [2]. In rats with unilateral lesion of the nigrostriatal tract, BU224 (14 mg/kg) significantly increased ipsiversive rotations [3]. In rats with warm water tail withdrawal procedure, BU224 inhibited the enhancement of morphine and tramadol antinociception induced by 2-BFI and agmatine [4].
References:
[1]. Hudson AL, Gough R, Tyacke R, et al. Novel selective compounds for the investigation of imidazoline receptors. Ann N Y Acad Sci, 1999, 881: 81-91.
[2]. Finn DP, Martí O, Harbuz MS, et al. Behavioral, neuroendocrine and neurochemical effects of the imidazoline I2 receptor selective ligand BU224 in naive rats and rats exposed to the stress of the forced swim test. Psychopharmacology (Berl), 2003, 167(2): 195-202.
[3]. Macinnes N, Duty S. Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway. Br J Pharmacol, 2004, 143(8): 952-959.
[4]. Thorn DA, Zhang Y, Peng BW, et al. Effects of imidazoline I₂ receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol, 2011, 670(2-3): 435-440.
| Physical Appearance | White solid |
| Storage | Desiccate at RT |
| M.Wt | 233.7 |
| Cas No. | 205437-64-5 |
| Formula | C12H11N3·HCl |
| Solubility | insoluble in DMSO |
| Chemical Name | 2-(4,5-dihydro-1H-imidazol-2-yl)quinoline hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | C1NC(c2nc(cccc3)c3cc2)=NC1.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure