ADC Linker
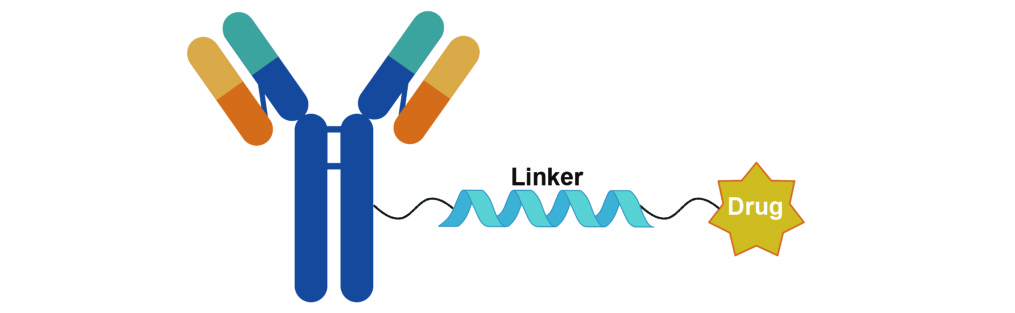

Antibody–drug conjugate (ADC) linkers are core functional components of ADCs, serving as covalent bridges that connect targeting antibodies to cytotoxic payloads. Their design is governed by the dual requirement of circulatory stability and efficient intracellular release. By employing specific chemical structures (such as peptide bonds, disulfide bonds, and acid-labile linkages), linkers enable precise control over ADC behavior: they preserve the integrity of the conjugate in the bloodstream to prevent premature payload release and off-target toxicity, while, after cellular uptake (e.g., via endocytosis and subsequent lysosomal degradation), they facilitate efficient payload liberation through enzymatic hydrolysis, reductive cleavage, or pH-responsive mechanisms. In this way, linkers form the molecular basis for both the targeting selectivity and safety profile of ADCs.
As essential reagents for ADC research and development, structurally diverse linkers provide critical support for both basic research and translational drug discovery. Different linker types (including cleavable, non-cleavable, and hydrophilically modified linkers) can be tailored to a wide range of payloads, such as microtubule inhibitors and DNA-damaging agents, thereby accommodating diverse molecular targets and heterogeneous tumor microenvironments. Linker stability, intracellular release efficiency, and biocompatibility directly shape the therapeutic window of ADCs and contribute to overcoming the resistance and toxicity limitations associated with conventional chemotherapy. In target validation, structure–activity relationship studies of ADCs, and preclinical drug screening, linker reagents thus provide crucial technical leverage for the precise modulation of ADC performance and for expanding the therapeutic applications of ADCs in oncology.
-
 BA2580 Propargyl-PEG2-NHBocSummary: Propargyl-PEG2-NHBoc is a degradable (cleavable) linker for the synthesis of antibody coupled active molecules (ADCs).
BA2580 Propargyl-PEG2-NHBocSummary: Propargyl-PEG2-NHBoc is a degradable (cleavable) linker for the synthesis of antibody coupled active molecules (ADCs). -
 BA2587 Fmoc-NH-PEG6-CH2COOHSummary: Fmoc-NH-PEG6-CH2COOH is a degradable ADClinker for antibody active molecule coupler (ADC) synthesis.
BA2587 Fmoc-NH-PEG6-CH2COOHSummary: Fmoc-NH-PEG6-CH2COOH is a degradable ADClinker for antibody active molecule coupler (ADC) synthesis. -
 BA2613 Fmoc-Gly-Gly-Phe-OtBuSummary: A commonly used degradable (cleavable) linker for antibody-coupled reactive molecules (ADCs).
BA2613 Fmoc-Gly-Gly-Phe-OtBuSummary: A commonly used degradable (cleavable) linker for antibody-coupled reactive molecules (ADCs). -
 BA2624 CL2A-SN-38Summary: CL2A-SN-38 is, linked by linkerCL2A and toxic molecule SN-38, which can be used to prepare antibody coupled active molecules (ADCs).
BA2624 CL2A-SN-38Summary: CL2A-SN-38 is, linked by linkerCL2A and toxic molecule SN-38, which can be used to prepare antibody coupled active molecules (ADCs). -
 BA2627 McMMAFSummary: McMMAF is composed of the highly efficient microtubule inhibitor MonomethylF (MMAF) coupled with the protecting group maleimidocaproyl, which can be used as the active molecular linker.
BA2627 McMMAFSummary: McMMAF is composed of the highly efficient microtubule inhibitor MonomethylF (MMAF) coupled with the protecting group maleimidocaproyl, which can be used as the active molecular linker. -
 BA2628 SMCC-DM1Summary: SMCC-DM1 (DM1-SMCC) is formed by linking the linkerSMCC to the toxic molecule DM1 and can be used to prepare antibody-coupled active molecules.
BA2628 SMCC-DM1Summary: SMCC-DM1 (DM1-SMCC) is formed by linking the linkerSMCC to the toxic molecule DM1 and can be used to prepare antibody-coupled active molecules. -
 BA2633 MC-VA-PAB-ExatecanSummary: MC-VA-PAB-Exatecan is part of an antibody-coupled active molecule.
BA2633 MC-VA-PAB-ExatecanSummary: MC-VA-PAB-Exatecan is part of an antibody-coupled active molecule. -
 BA2636 Mal-PEG8-Val-Ala-PAB-ExatecanSummary: Mal-PEG8-Val-Ala-PAB-Exatecan is an antibody-active molecule coupling linker.
BA2636 Mal-PEG8-Val-Ala-PAB-ExatecanSummary: Mal-PEG8-Val-Ala-PAB-Exatecan is an antibody-active molecule coupling linker. -
 BA2643 DM21Summary: DM21 is a cleavable affix of an ADC active molecule and linker (ADClinker).
BA2643 DM21Summary: DM21 is a cleavable affix of an ADC active molecule and linker (ADClinker). -
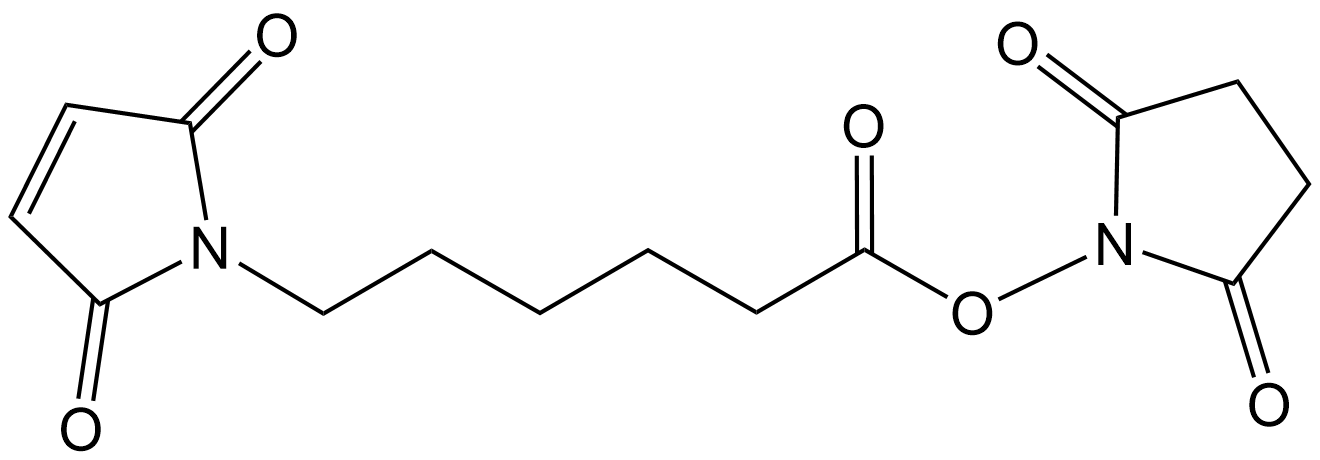 C8736 N-(6-Maleimidocaproyloxy)succinimideSummary: A heterobifunctional crosslinker commonly used in biomedical research
C8736 N-(6-Maleimidocaproyloxy)succinimideSummary: A heterobifunctional crosslinker commonly used in biomedical research


