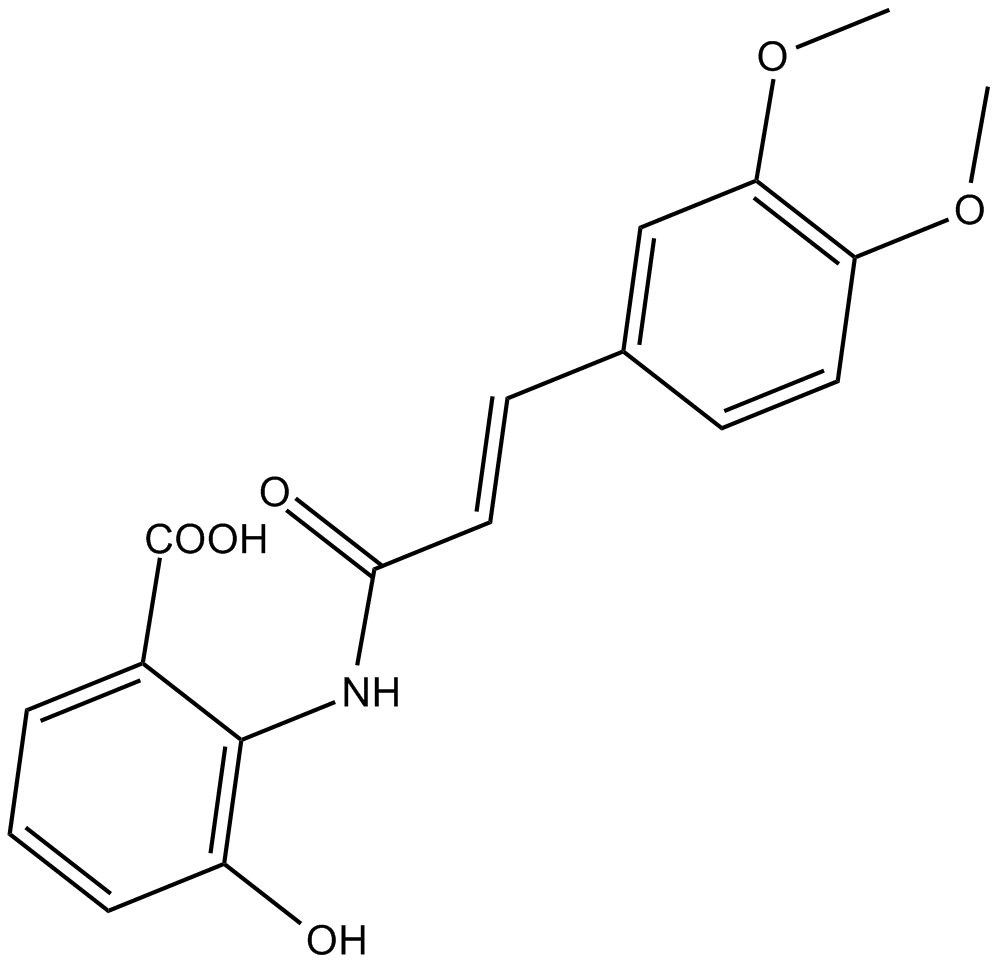
3,4-DAA
N-(3,4,-Dimethoxycinnamoyl) anthranilic acid (3,4-DAA) is a synthetic derivative of the tryptophan metabolite anthranilic acid [1].
Degradation of the essential amino acid Trp by indoleamine 2,3-dioxygenase (IDO) plays an important role in immunity. IDO has been implicated in immune modulation through limiting T cell function and engage mechanisms of immune tolerance. Activation of IDO has been observed during tumor development, helping malignant cells escape eradication by the immune system [2].
3,4-DAA suppressed antigen-specific proliferation of MBP Ac1-11 TCR transgenic CD4+ T cells by arrested the cells in G1/S-phase. 3,4-DAA (200 μM) reduced the release of IL-2, IFN-γ, and TNF-α and 3,4-DAA (30 μM)increased the level of IL-4 and IL-10 in splenocytes from MBP Ac1-11 TCR transgenic T cells after antigen stimulation [1]. 3,4-DAA dose-dependently decreased IFNγ–induced cell surface expression of MHC class II and costimulatory molecules and suppressed the expression of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) release from EOC20 cells and phosphorylation of STAT1α induced by IFNγ. In Mice with experimental autoimmune encephalomyelitis, oral administration of 3,4-DAA (300 mg/kg per day) exhibited fewer and milder relapses and less severe disease compared to control animals [1]. In allograft immunorejection model, administration of 3,4-DAA reduced histological severity of allograft immunorejection, decreased serum levels of TNF-α and IFN-γ, and raised serum levels of IL-10 [3].
References:
[1] Platten M, Ho P P, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite[J]. Science, 2005, 310(5749): 850-855.
[2] Hirata F, Ohnishi T, Hayaishi O. Indoleamine 2, 3-Dioxygenase[J]. J. Biol. Chem, 1977, 252: 4637.
[3] Sun Q F, Ding J G, Sheng J F, et al. Novel action of 3, 4‐DAA ameliorating acute liver allograft injury[J]. Cell biochemistry and function, 2011, 29(8): 673-678.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 343.3 |
| Formula | C18H17NO6 |
| Solubility | Soluble in DMSO |
| Chemical Name | 2-[3-(3,4-dimethoxy-phenyl)-acryloylamino]-3-hydroxy-benzoic acid |
| SDF | Download SDF |
| Canonical SMILES | COc(ccc(/C=C/C(Nc(c(O)ccc1)c1C(O)=O)=O)c1)c1OC |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
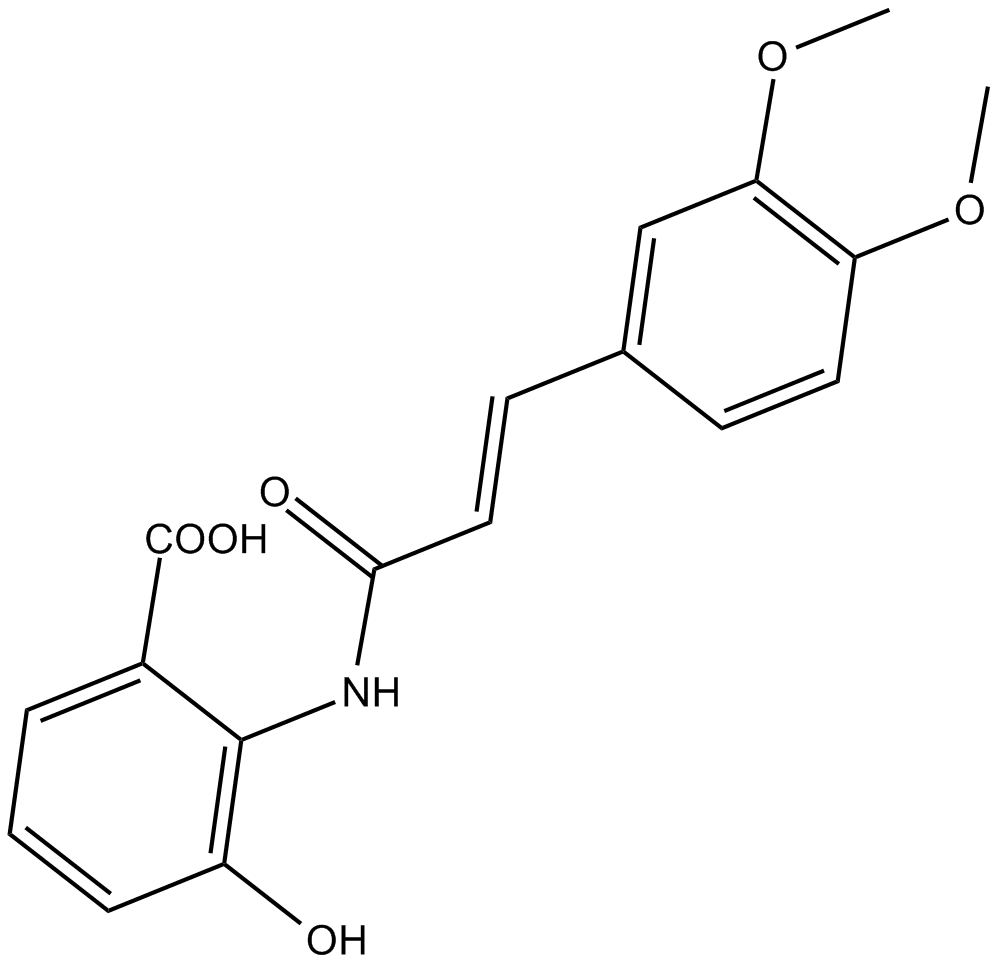
Chemical structure
Related Biological Data