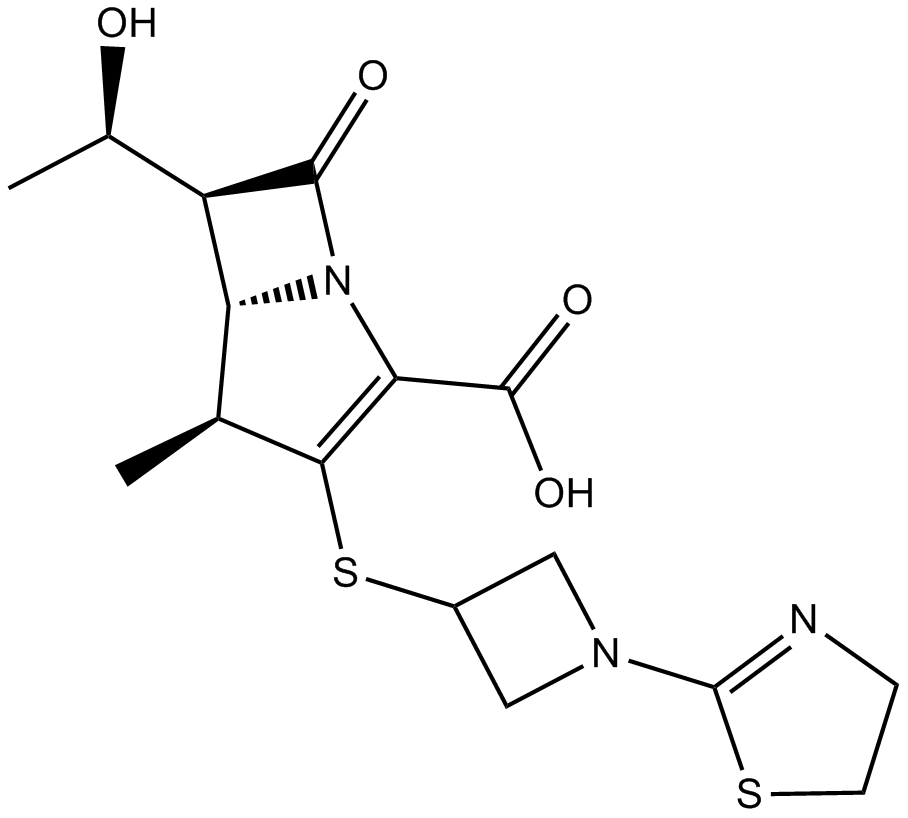
Tebipenem
Tebipenem is an orally available carbapenem antibiotic. Tebipenem is active against a panel of clinical isolates from a variety of bacterial species (MIC50s ≤ 0.0039 ~ 8 µg/ml), including methicillin-resistant strains of Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis), as well as penicillin-resistant Streptococcus pneumoniae (S. pneumonia). Tebipenem also inhibits β-lactamase in a time- and concentration-dependent manner. Tebipenem pivoxil, a derivative of tebipenem, has been under development as the first orally available carbapenem antibiotic for the treatment of respiratory and otolaryngological infections caused by drug-resistant S. pneumonia in pediatric patients.
References:
1. Hazra S, Xu H, Blanchard JS. Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry, 2014, 53(22): 3671-3678.
2. Fujimoto K, Takemoto K, Hatano K, et al. Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals. Antimicrobial Agents and Chemotherapy, 2013, 57(2): 697-707.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 383.49 |
| Cas No. | 161715-21-5 |
| Formula | C16H21N3O4S2 |
| Solubility | insoluble in EtOH; ≥19.15 mg/mL in H2O with gentle warming; ≥24.9 mg/mL in DMSO |
| Chemical Name | (4R,5S,6S)-3-[1-(4,5-dihydro-1,3-thiazol-2-yl)azetidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | C[C@H]([C@H]([C@@H]([C@H]1C)N2C(C(O)=O)=C1SC(C1)CN1C1=NCCS1)C2=O)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Animal experiment:[2] | |
|
Animal models |
A mouse model of penicillin-resistant S. pneumoniae infection |
|
Dosage form |
0.32 ~ 3.2 mg/kg Administered intravenously thrice daily 1 day and 2 days after infection |
|
Applications |
Tebipenem dose-dependently decreased the number of colony forming units (CFUs) in the lungs of infected mice. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Hazra S, Xu H, Blanchard JS. Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry, 2014, 53(22): 3671-3678. 2. Fujimoto K, Takemoto K, Hatano K, et al. Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals. Antimicrobial Agents and Chemotherapy, 2013, 57(2): 697-707. |
|
Quality Control & MSDS
- View current batch:
Chemical structure