Search results for: 'staurosporine cgp 41251'
-
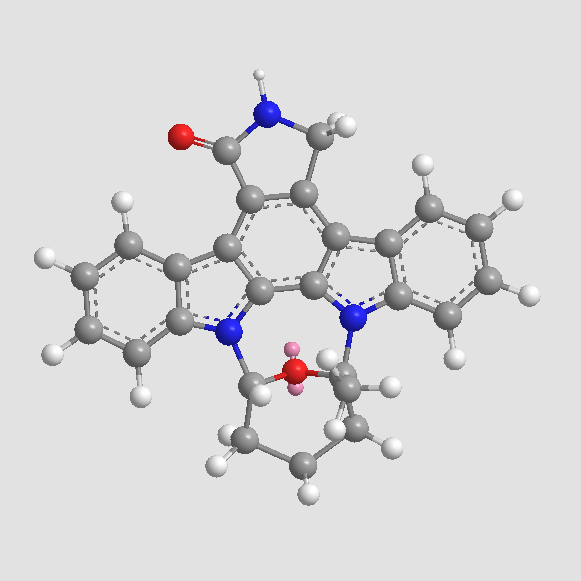 A8192 Staurosporine20 CitationSummary: serine/threonine protein kinases inhibitor
A8192 Staurosporine20 CitationSummary: serine/threonine protein kinases inhibitor -
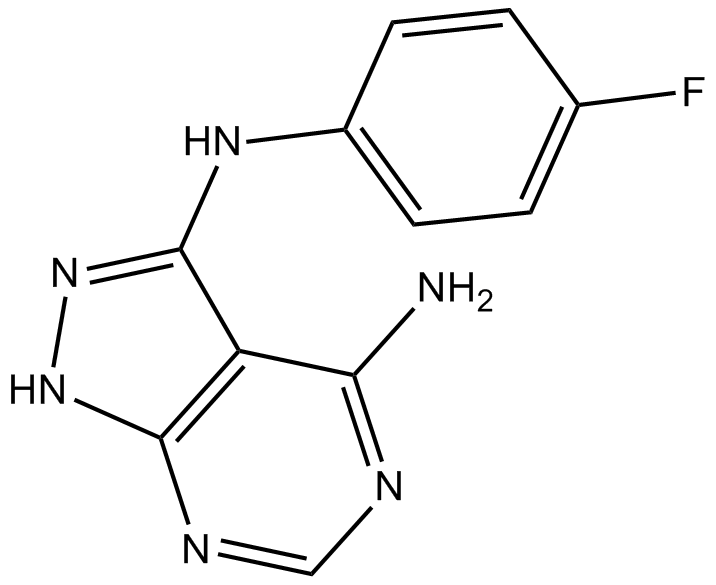 B3570 CGP 57380Target: MNKSummary: MNK1 inhibitor, specific and selective
B3570 CGP 57380Target: MNKSummary: MNK1 inhibitor, specific and selective -
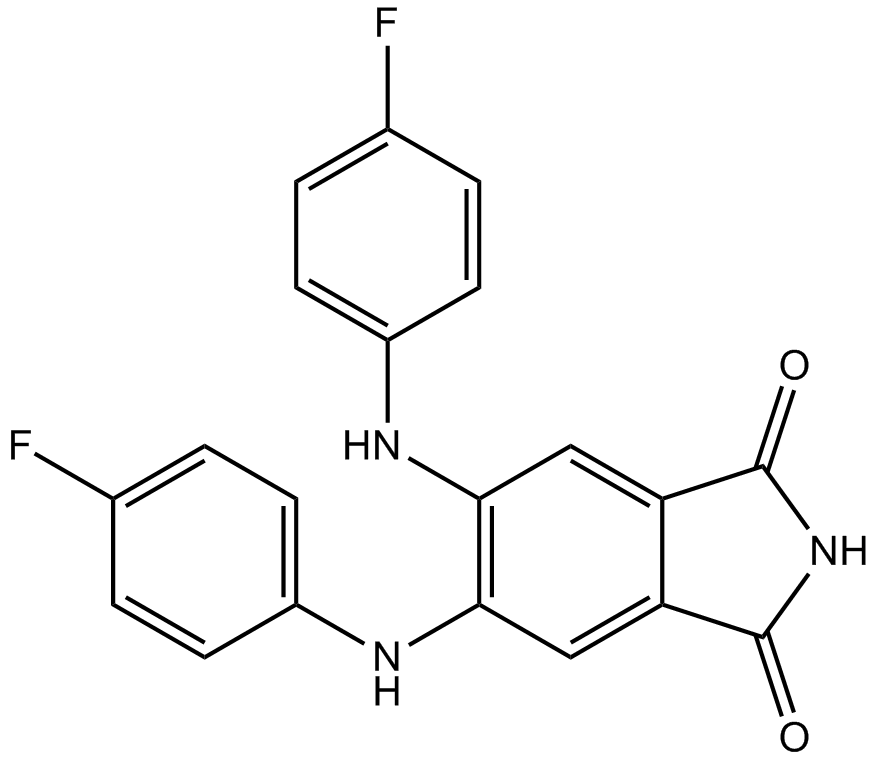 B7056 CGP 53353Summary: PKCβII inhibitor
B7056 CGP 53353Summary: PKCβII inhibitor -
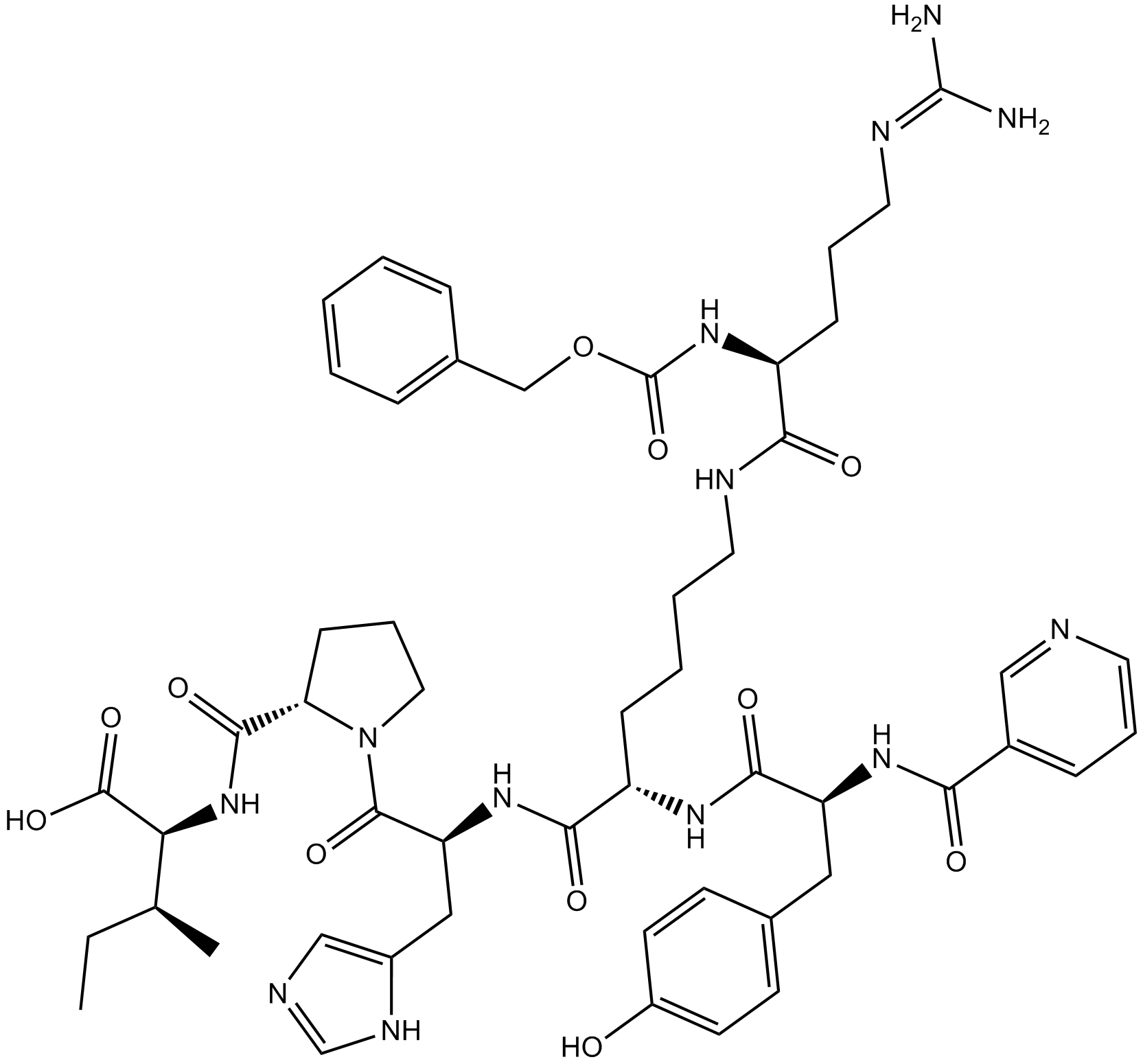 B5316 CGP 42112Summary: angiotensin AT2 receptor ligand
B5316 CGP 42112Summary: angiotensin AT2 receptor ligand -
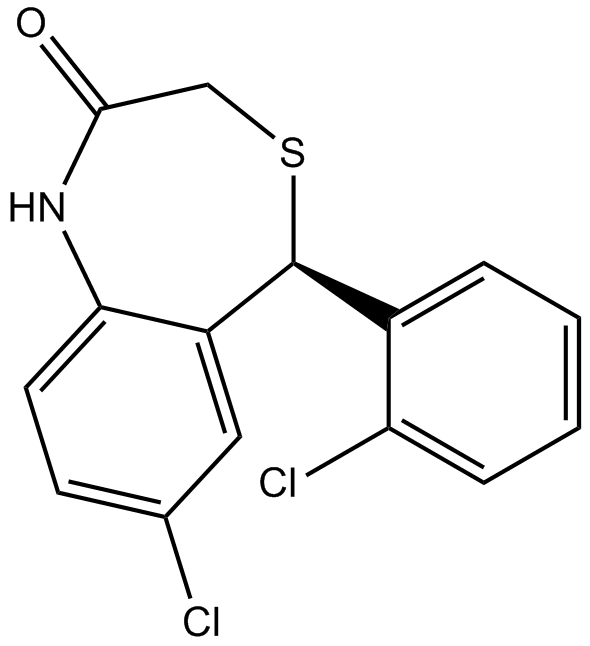 B6602 CGP 37157Summary: Antagonist of mitochondrial Na+-Ca2+ exchanger
B6602 CGP 37157Summary: Antagonist of mitochondrial Na+-Ca2+ exchanger -
 B6654 CGP 35348Summary: Selective GABAB antagonist, brain penetrant
B6654 CGP 35348Summary: Selective GABAB antagonist, brain penetrant -
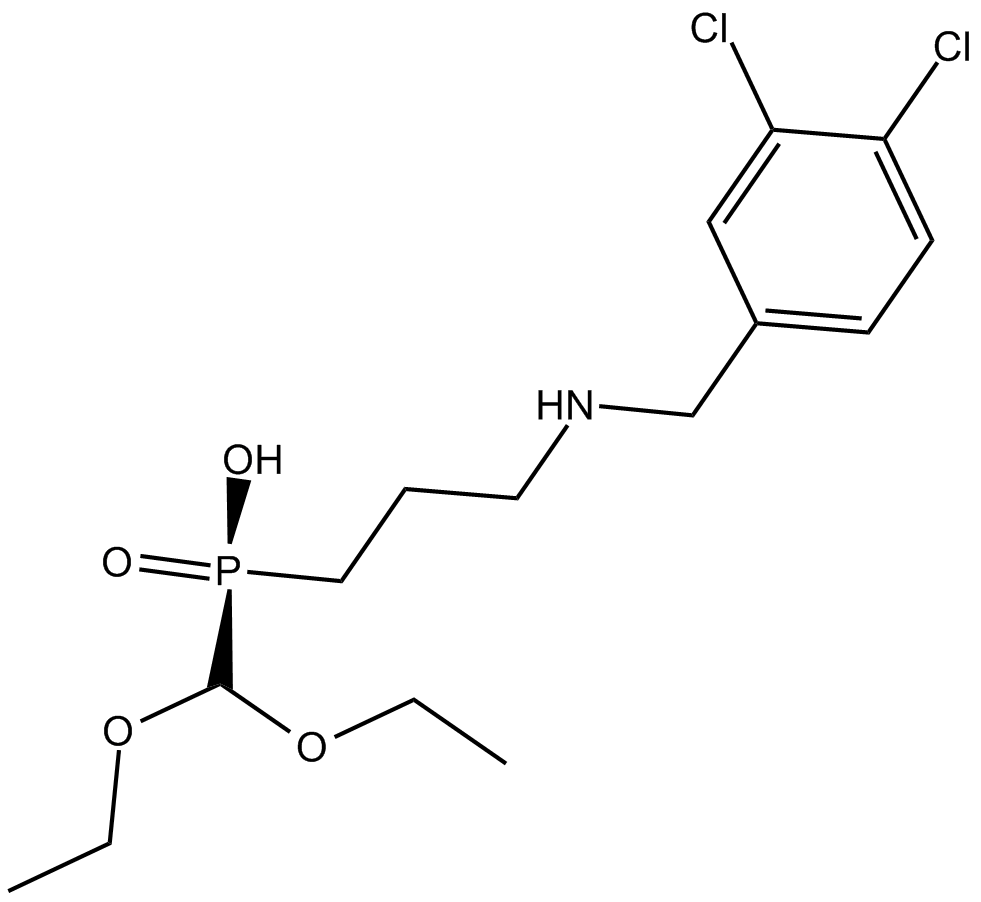 B6655 CGP 52432Summary: GABAB receptor antagonist
B6655 CGP 52432Summary: GABAB receptor antagonist -
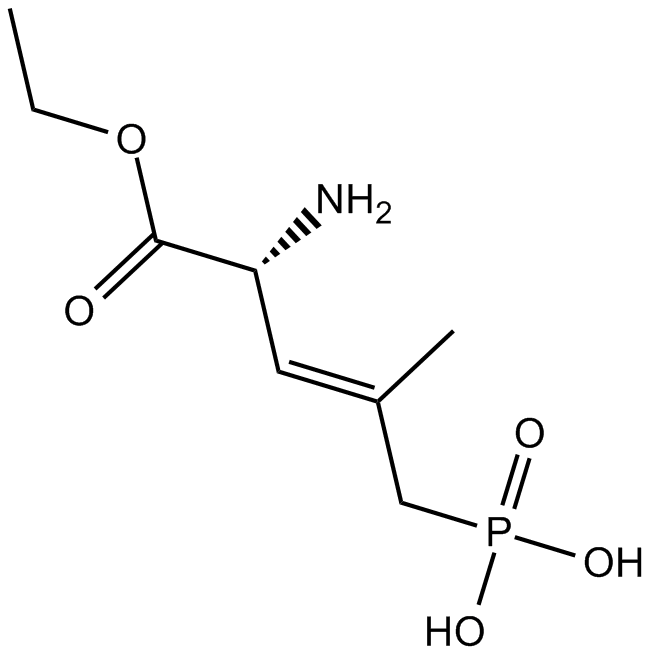 B6726 CGP 39551Summary: NMDA antagonist
B6726 CGP 39551Summary: NMDA antagonist -
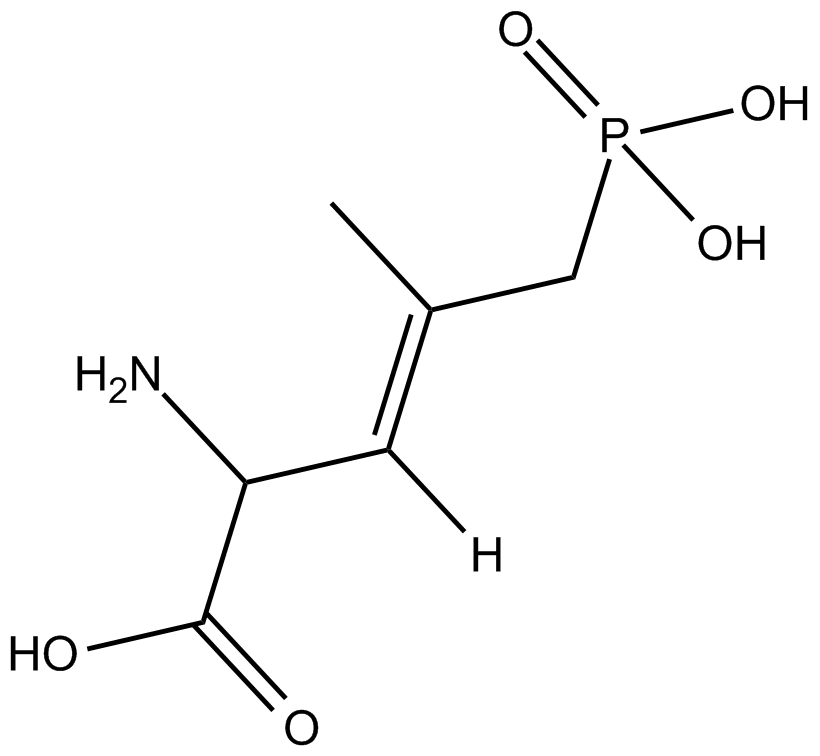 B6751 CGP 37849Summary: NMDA receptor antagonist
B6751 CGP 37849Summary: NMDA receptor antagonist -
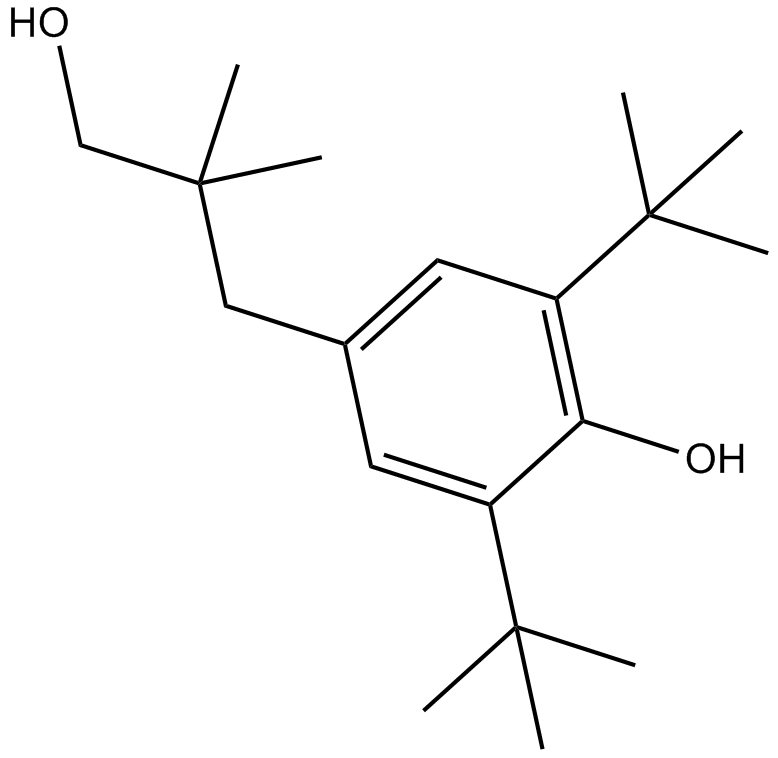 B6771 CGP 7930Summary: Positive allosteric modulator of GABAB receptors
B6771 CGP 7930Summary: Positive allosteric modulator of GABAB receptors


