Puromycin aminonucleoside
Puromycin aminonucleoside (CAS 58-60-6) is the aminonucleoside moiety derived from the antibiotic puromycin. It induces nephrotic injury in experimental animal models, leading to significant proteinuria and structural alterations in renal glomeruli. In vitro, treatment with puromycin aminonucleoside alters podocyte morphology, characterized by reductions in cellular microvilli and disruption of foot-process structures. In vivo administration in rats induces glomerular lesions resembling focal segmental glomerulosclerosis (FSGS) and lipid accumulation in mesangial cells. This compound serves primarily as an investigative tool in elucidating mechanisms underlying nephrotic syndrome.
- 1. Tingting Li, Chonghui Hu, et al. "Cancer-Associated Fibroblasts Foster a High-Lactate Microenvironment to Drive Perineural Invasion in Pancreatic Cancer." Cancer Res MARCH 26 2025
- 2. Molly E. Drosen, Sarojini Bulbule, et al. "Inactivation of ATG13 stimulates chronic demyelinating pathologies in muscle-serving nerves and spinal cord." Immunol Res. 2025 Jan 7;73(1):27 PMID: 39777574
- 3. Shuhui Zhou, Liping Zheng, et al. "Shensu IV maintains the integrity of the glomerular filtration barrier and exerts renal protective effects by regulating endogenous hydrogen sulfide levels." Front. Pharmacol. , 09 December 2024 Sec. Ethnopharmacology
- 4. Tao Ma, Wei Huang, et al. "AIBP protects drug-induced liver injury by inhibiting MAPK-mediated NR4A1 expression." Volume 27, Issue 10110873 October 18, 2024
- 5. Shuye Lin, Hanli Xu, et al. "UHRF1/DNMT1–MZF1 axis-modulated intragenic site-specific CpGI methylation confers divergent expression and opposing functions of PRSS3 isoforms in lung cancer." Acta Pharm Sin B. 2023 May;13(5):2086-2106. PMID: 37250150
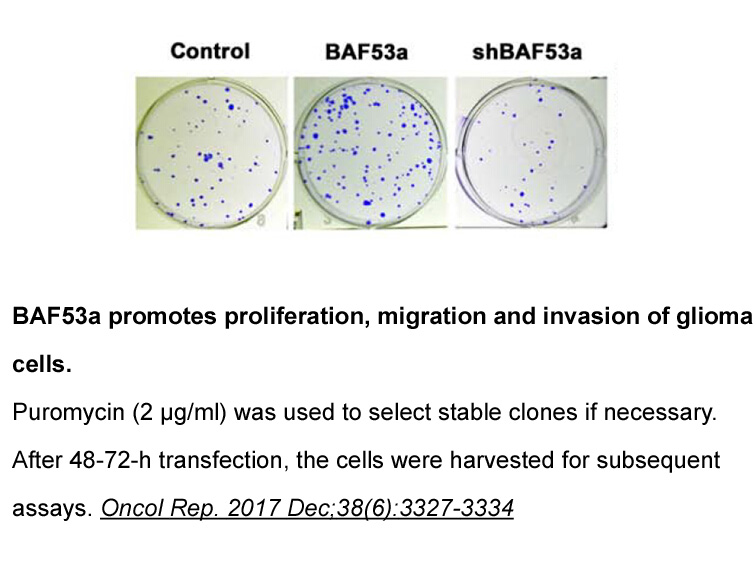
- 6. Meng L, Wang X, et al. "BAF53a is a potential prognostic biomarker and promotes invasion and epithelial-mesenchymal transition of glioma cells." Oncol Rep. 2017 Dec;38(6):3327-3334. PMID:29039584
| Storage | Store at -20°C |
| M.Wt | 294.31 |
| Cas No. | 58-60-6 |
| Formula | C12H18N6O3 |
| Synonyms | 3'-Amino-3'-deoxy-N6,N6-dimethyladenosine |
| Solubility | ≥14.45 mg/mL in DMSO; ≥29.4 mg/mL in EtOH with gentle warming; ≥29.5 mg/mL in H2O with gentle warming |
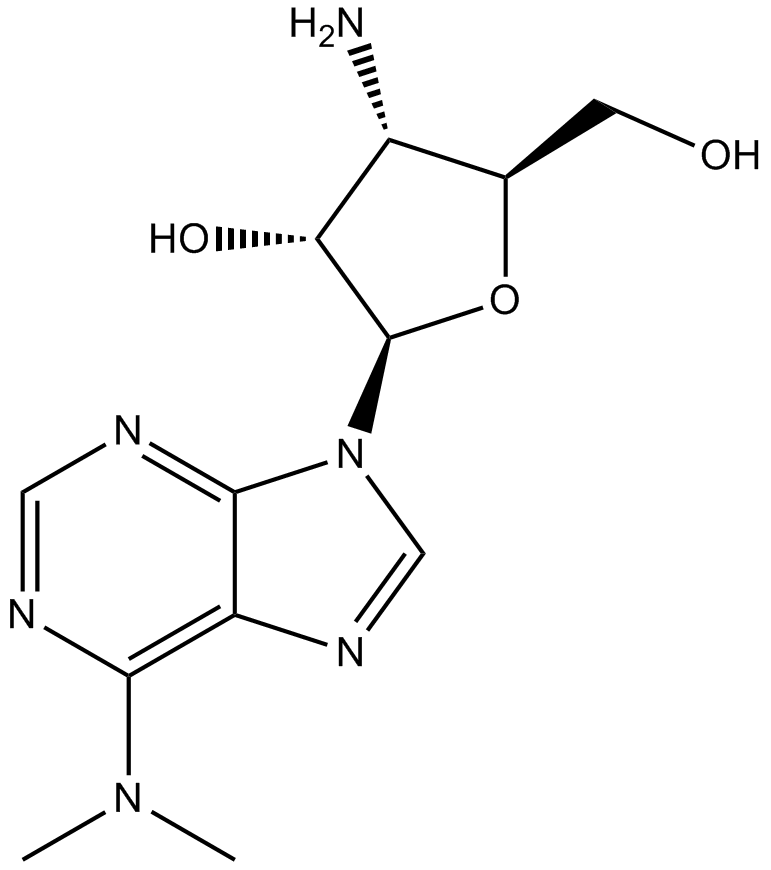
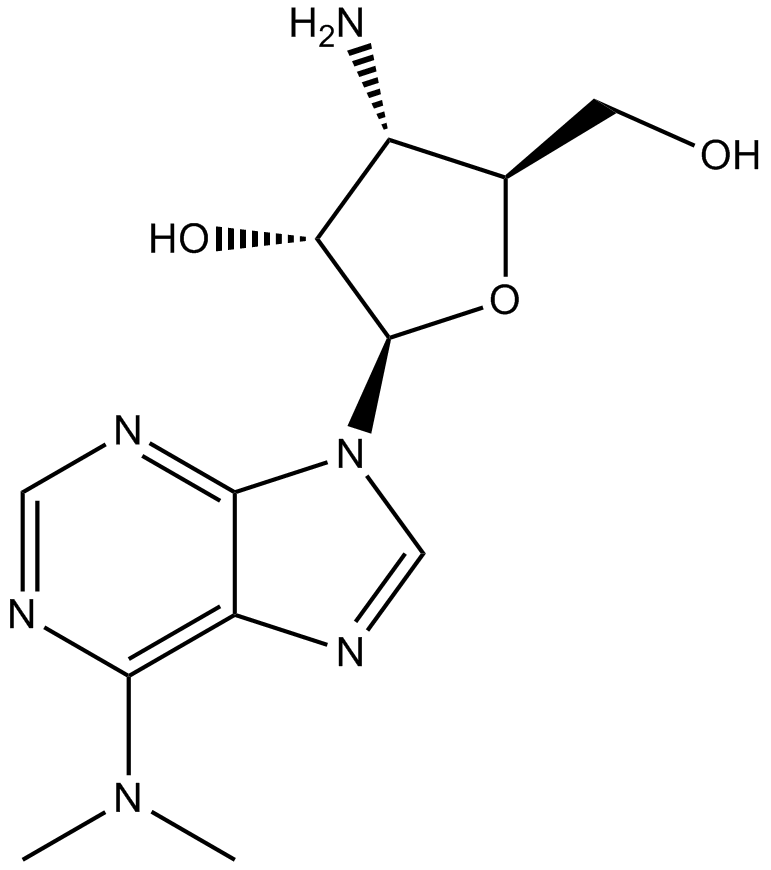
| Chemical Name | 4-amino-2-[6-(dimethylamino)purin-9-yl]-5-(hydroxymethyl)oxolan-3-ol |
| SDF | Download SDF |
| Canonical SMILES | CN(C)c1c2nc[n](C(C3O)OC(CO)C3N)c2ncn1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Madin-Darby canine kidney (MDCK) cells |
|
Preparation method |
The solubility of this compound in DMSO is >14.5mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
48 h |
|
Applications |
In vector- and PMAT-transfected MDCK cells, Puromycin aminonucleoside (PAN) exhibited cell cytotoxicity with the IC50 values of 48.9 ± 2.8 and 122.1 ± 14.5 μM, respectively. PAN (250 μM) was toxic to both PMAT-expressing and vector-transfected cells. Puromycin aminonucleoside uptake in PMAT-expressing cells was four fold higher at pH 6.6 than that at pH 7.4. |
| Animal experiment [2,3]: | |
|
Animal models |
Nephrosis rats |
|
Dosage form |
Intravenous injection, 60 mg/kg, 150 mg/kg |
|
Application |
In nephrosis rats, the number of podocytes per glomerulus was 90.7 on Day 4 in PAN (8 mg/100 g, i.v.) treated group. The amount of nephrin per glomerulus in PAN-treated nephrosis rats reduced to 0.46 ± 0.06 fmol and 0.35±0.04 fmol on Day 4 and Day 7. The nephrin amount per podocyte was significantly decreased association with the development of proteinuria in Puromycin aminonucleoside nephrosis rats. Rats given PAN (100 mg/kg, s.c.) gained less weight and their serum creatinine levels were higher than the control rats, indicating Puromycin aminonucleoside impaired renal function. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Xia L, Zhou M, Kalhorn T F, et al. Podocyte-specific expression of organic cation transporter PMAT: implication in puromycin aminonucleoside nephrotoxicity. American Journal of Physiology-Renal Physiology, 2009, 296(6): F1307-F1313. [2]. Kawakami, Hirotaka, et al. Dynamics of absolute amount of nephrin in a single podocyte in puromycin aminonucleoside nephrosis rats calculated by quantitative glomerular proteomics approach with selected reaction monitoring mode. Nephrology Dialysis Transplantation 27.4 (2011): 1324-1330. [3]. Nosaka, Kazuo, et al. An adenosine deaminase inhibitor prevents puromycin aminonucleoside nephrotoxicity. Free Radical Biology and Medicine 22.4 (1997): 597-605. |
|
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data