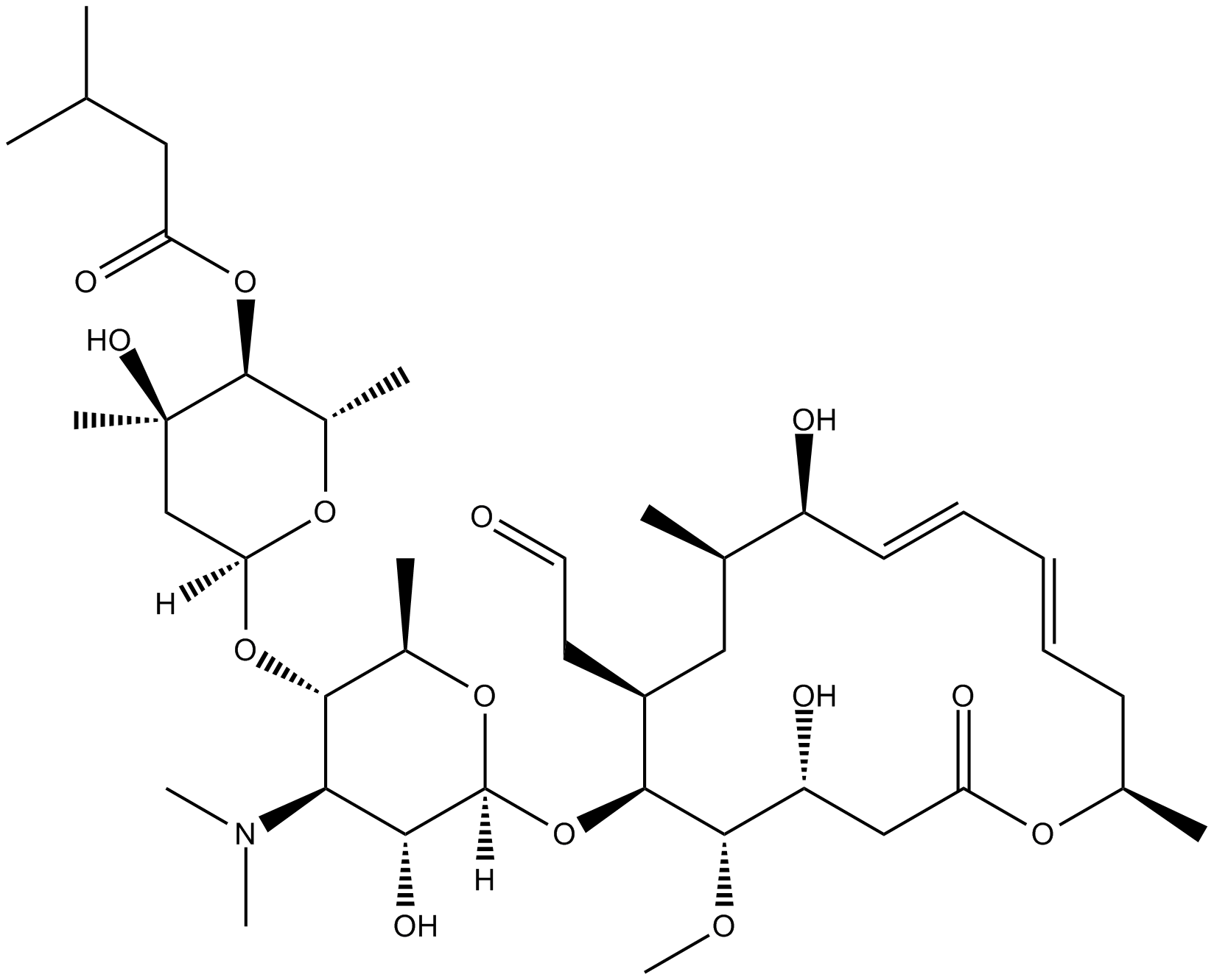
Leucomycin A1
Leucomycin A1, a major metabolite extracted from the leucomycin complex, is a major macrocyclic lactone antibiotics produced by Streptomyces kitasatoensis.
Leucomycin A1 is one of the more potent members of leucomycin complex. Leucomycin complex (kitasamycin) is effective against a wide spectrum of pathogens, such as Gram-positive bacteria, Gram-negative cocci, mycoplasma, and Leptospira. Leucomycin complex has been used as an animal health product for control of Gram positive bacteria, Gram negative cocci, mycoplasma, and Leptospira. Until now, little is known about the activity of individual analogues within the complex. At a concentration of 1.56μg/ml, kitasamycin inhibited all isolates of Diplococcus pneumonia [2]. Each component of leucomycins potently inhibited the growth of Gram-positive bacteria and showed not so strong activity against the Gram-negative bacteria. Each component showed the same tendency towards the antibacterial spectrum [3].
References:
[1] Hata T, Sano Y, Ohki N, et al. Leucomycin, a new antibiotic[J]. The Journal of antibiotics, 1953, 6(2): 87-89.
[2] Balducci Y, BODEY G P. In vitro activity of kitasamycin against gram-positive cocci[J]. The Journal of antibiotics, 1974, 27(7): 516-519.
[3] Omura S, Katagiri M, Umezawa I, et al. Structure-biological activities relationships among leucomycins and their derivatives[J]. The Journal of antibiotics, 1968, 21(9): 532-538.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 786.0 |
| Cas No. | 16846-34-7 |
| Formula | C40H67NO14 |
| Synonyms | Kitasamycin A1,3-deacetyl Josamycin,Leucomycin V 4-isovalerate,9-dihydro Niddamycin,Turimycin H5 |
| Solubility | Soluble in DMSO |
| Chemical Name | 4B-(3-methylbutanoate) leucomycin V |
| SDF | Download SDF |
| Canonical SMILES | O=C1C[C@H]([C@@H]([C@H]([C@H](C[C@H]([C@H](/C=C/C=C/C[C@H](O1)C)O)C)CC=O)O[C@@]2(O[C@@H]([C@H]([C@@H]([C@H]2O)N(C)C)O[C@]3(C[C@@](O)([C@H]([C@@H](O3)C)OC(CC(C)C)=O)C)[H])C)[H])OC)O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure