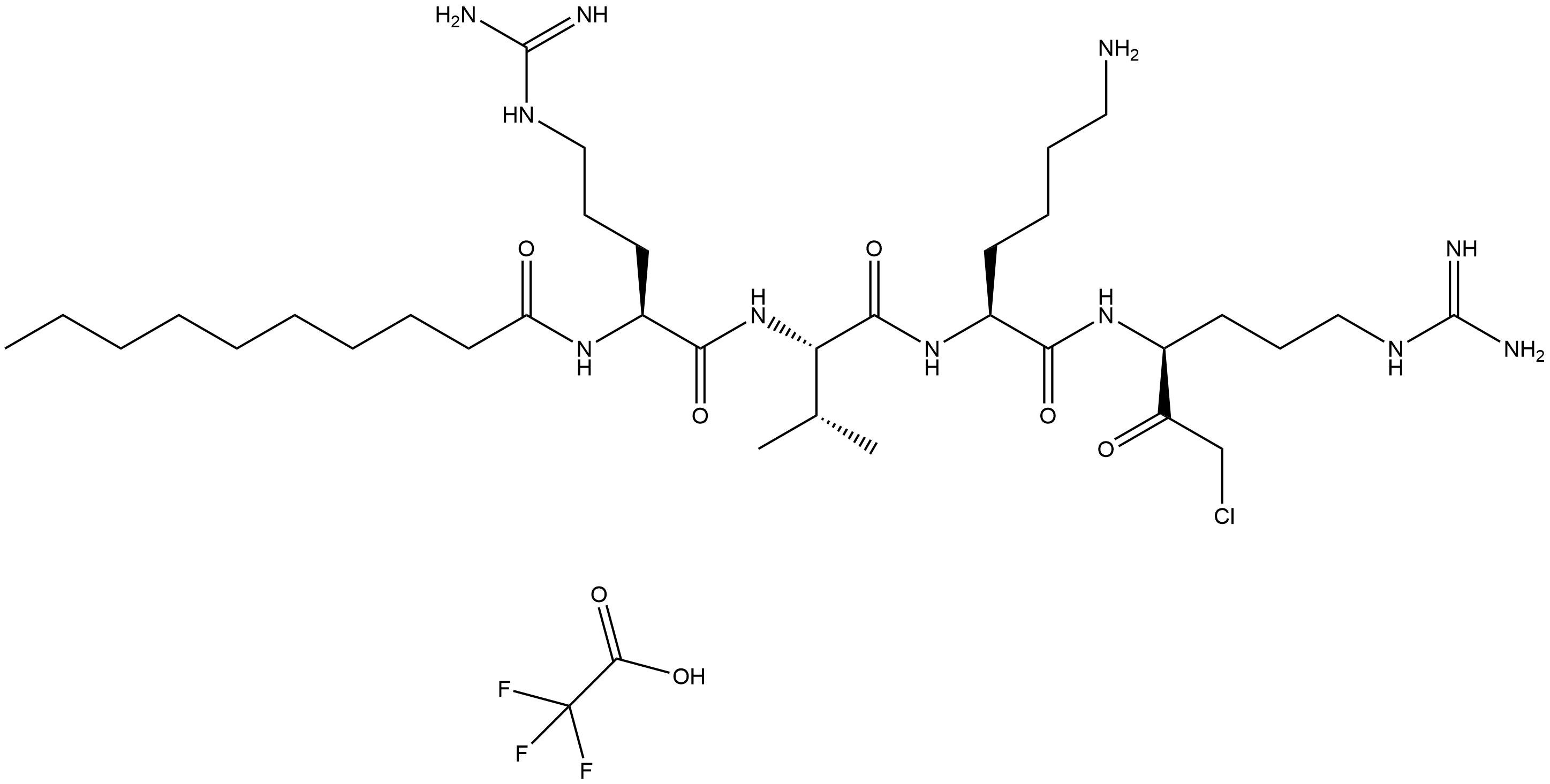
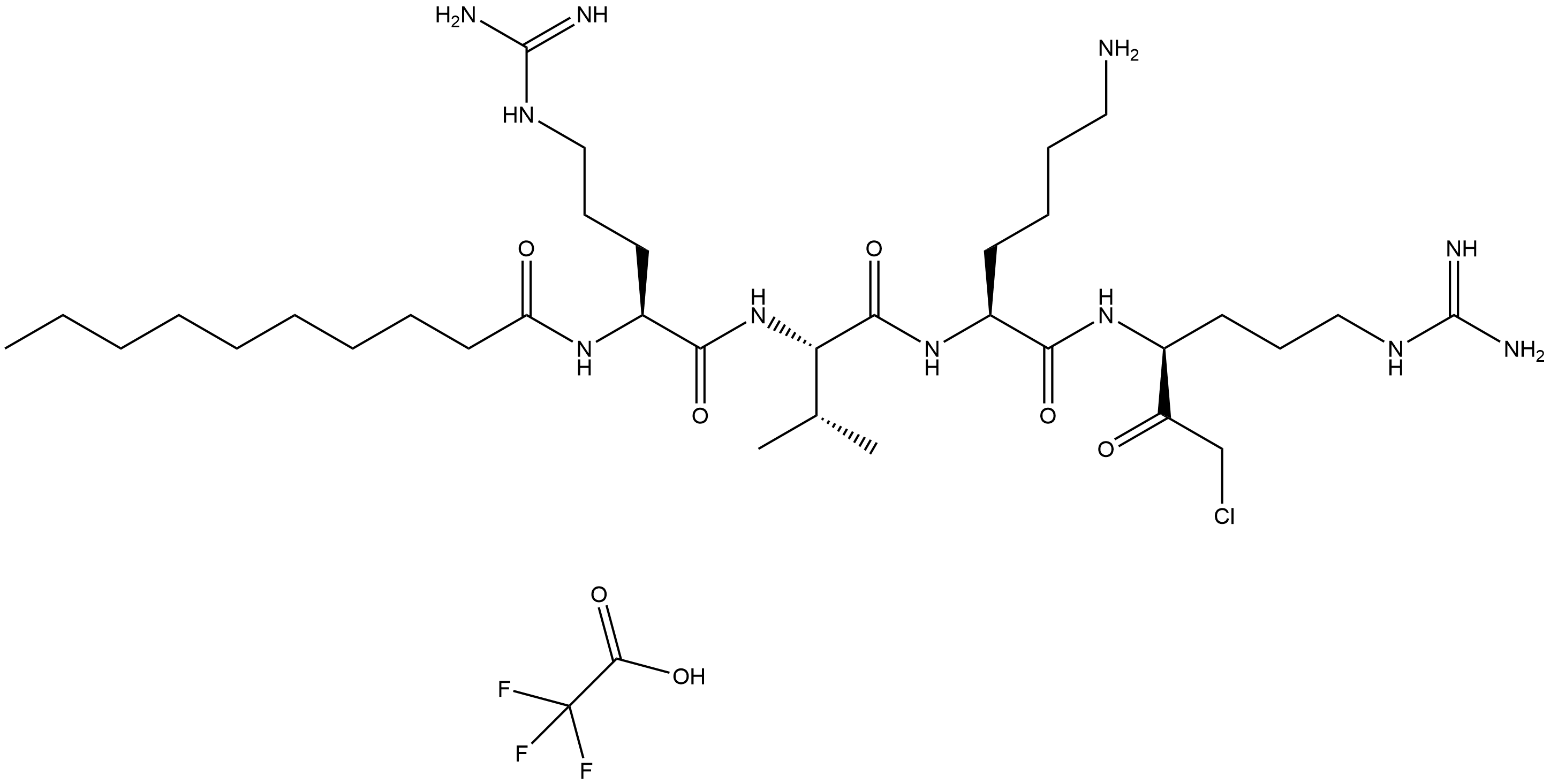
Decanoyl-RVKR-CMK TFA
Decanoyl-RVKR-CMK TFA (CAS No.: 2098497-25-5) is a synthetic peptide compound that functions as a proprotein convertase (PC) inhibitor. Its core structure consists of a decanoyl group (Decanoyl), the amino acid sequence RVKR (arginine - valine - lysine - arginine), and a chloromethyl ketone (CMK) moiety. The compound is converted into a trifluoroacetate (TFA) salt to enhance its water solubility and stability.Decanoyl-RVKR-CMK (Catalog No.B5437,CAS 150113-99-8) is a proprotein convertase (PC) inhibitor that targets enzymes crucial for generating bioactive peptides and activating various proteins involved in physiological and pathological processes, such as viral infections and cancer. In PC12 cells, treatment with decanoyl-RVKR-CMK (100 μM) partially blocked regulated VGF release at 48 hours and completely blocked it at 96 hours, without affecting basal VGF release; VGF processing was also reduced in treated cells and media. In K5-PACE4 transgenic mice, topical application of decanoyl-RVKR-CMK (300 μM for 2 days) inhibited TPA-induced epidermal proliferation. Prolonged treatment (100 μM daily for 3 weeks) led to fewer Ki-67-labeled basal keratinocytes in both transgenic and wild-type mouse epidermis, indicating reduced cell proliferation. These findings highlight decanoyl-RVKR-CMK’s potential in modulating PC activity and related biological processes.
References:
[1]. Fugère M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends in Pharmacological Sciences, 2005, 26(6): 294-301.
[2]. Garcia A L, Han S K, Janssen W G, et al. A prohormone convertase cleavage site within a predicted alpha-helix mediates sorting of the neuronal and endocrine polypeptide VGF into the regulated secretory pathway. Journal of Biological Chemistry, 2005, 280(50): 41595-41608.
[3]. Bassi D E, Zhang J, Cenna J, et al. Proprotein convertase inhibition results in decreased skin cell proliferation, tumorigenesis, and metastasis. Neoplasia, 2010, 12(7): 516-526.
| Storage | Store at -80°C |
| M.Wt | 858.45 |
| Cas No. | 2098497-25-5 |
| Formula | C36H67ClF3N11O7 |
| Synonyms | 4-MC;2-(4-Methoxybenzylidene) acetophenone;NSC636917 |
| Chemical Name | N-((6S,9S,12S,15S)-1,20-diamino-9-(4-aminobutyl)-6-(2-chloroacetyl)-1,20-diimino-12-isopropyl-8,11,14-trioxo-2,7,10,13,19-pentaazaicosan-15-yl)decanamide 2,2,2-trifluoroacetate |
| SDF | Download SDF |
| Canonical SMILES | CCCCCCCCCC(N[C@@H](CCCNC(N)=N)C(N[C@H](C(N[C@@H](CCCCN)C(N[C@H](C(CCl)=O)CCCNC(N)=N)=O)=O)C(C)C)=O)=O.FC(F)(F)C(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
Chemical structure