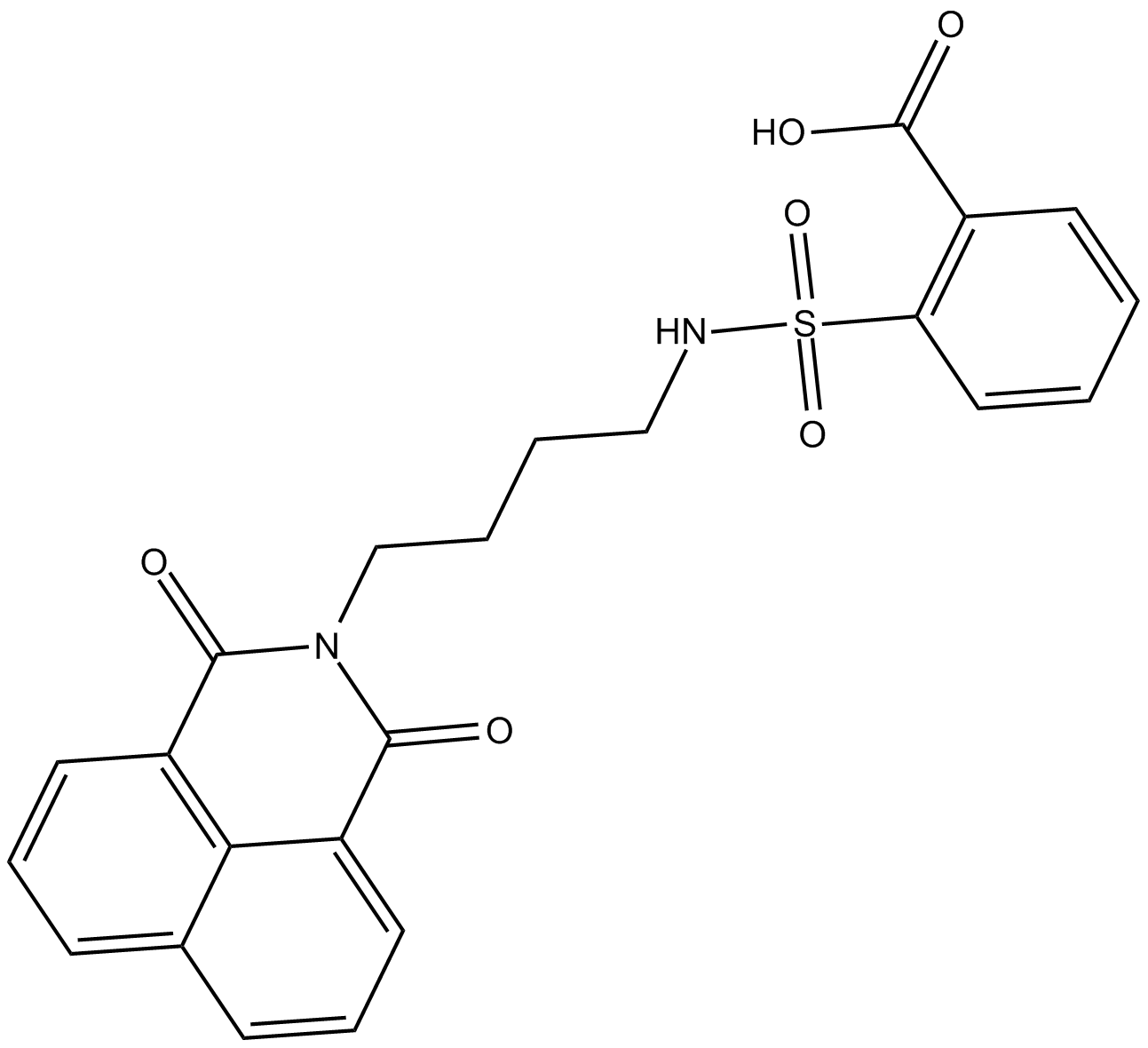
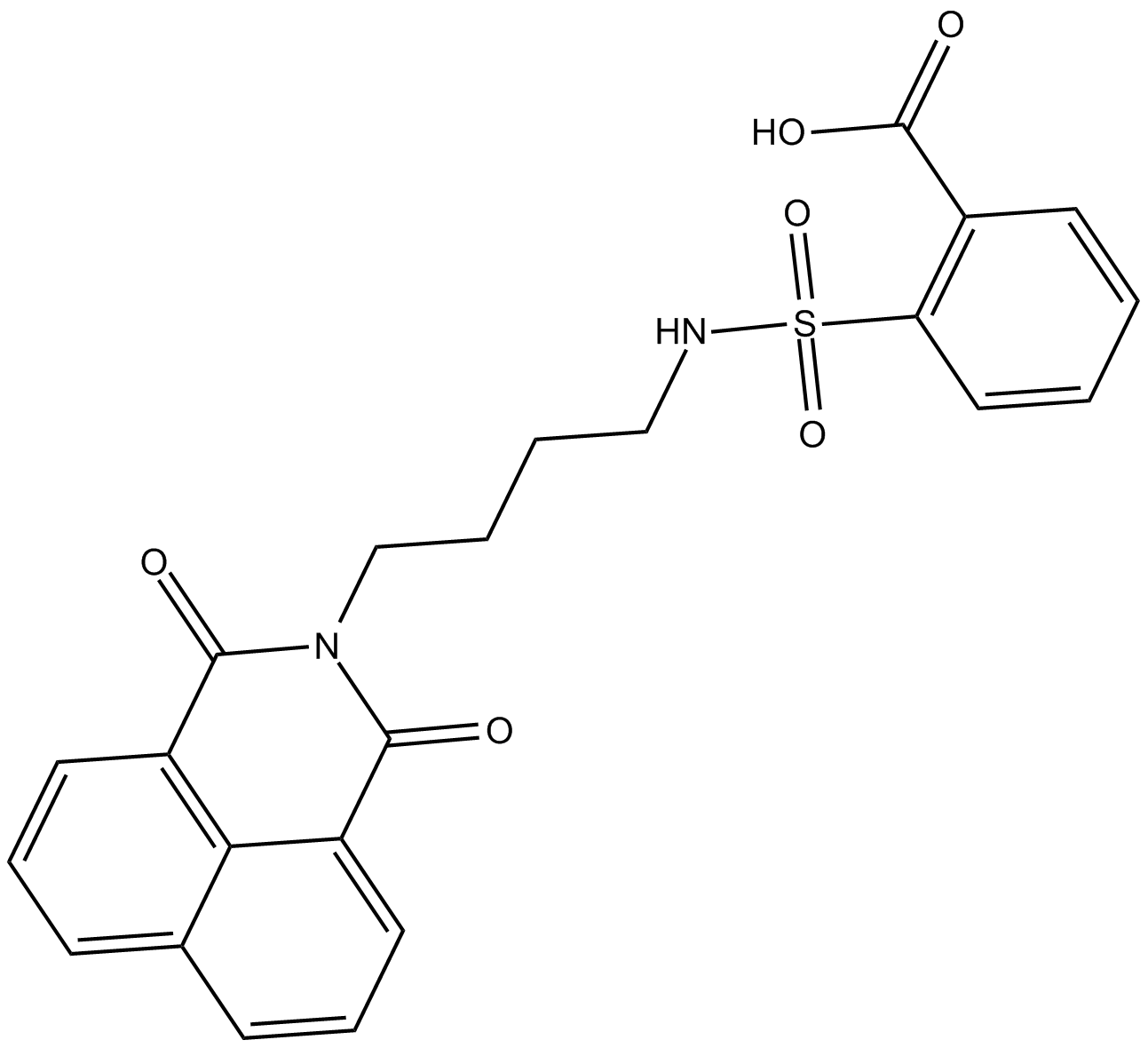
DBIBB
DBIBB, a butylsulfamoyl benzoic acid analog, is a non-lipid agonist of LPA2 with an EC50 of 0.10 μM. DBIBB has no effect at other LPA receptor subtypes [1]. The bioactive phospholipid lysophosphatidic acid (LPA) has been involved in stimulating cell proliferation, migration and survival by acting on its cognate G-protein-coupled receptors. Aberrant LPA production, receptor expression and signalling probably contribute to cancer initiation, progression and metastasis [2].
In vitro: DBIBB treatment postirradiation significantly (p< 0.01) increased the clonogenic survival of IEC-6 cells in the 2-6 Gy dose range. DBIBB reduced DNA fragmentation 4hr after irradiation in a dose dependent manner. DBIBB also reduced caspase 3/7 activity and DNA fragmentation in LPA2MEF treated with adriamycin. In purified CD34+ progenitor cells, DBIBB significantly increased the total number of colonies and specifically enhanced the survival of the granulocyte/macrophage lineages [1].
In vivo: Using a murine GI-ARS mice model of partial-body irradiation (PBI) with shielding of the bone marrow contained in the tibiae, fibulae, and paws, administrations of up to 10 mg/kg of DBIBB for 10 days showed no visually observable adverse effects and pathological findings at necropsy, indicating the lack of toxicity. The group that received 10 mg/kg DBIBB showed a significant increase in survival. In C57BL/6 mice, DBIBB showed a dose-dependent increase in the number of surviving crypts compared with the vehicle control [1].
References:
[1] Patil R, Szabó E, Fells J I, et al. Combined Mitigation of the Gastrointestinal and Hematopoietic Acute Radiation Syndromes by an LPA 2 Receptor-Specific Nonlipid Agonist[J]. Chemistry & biology, 2015, 22(2): 206-216.
[2] Mills G B, Moolenaar W H. The emerging role of lysophosphatidic acid in cancer[J]. Nature Reviews Cancer, 2003, 3(8): 582-591.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 452.5 |
| Cas No. | 1569309-92-7 |
| Formula | C23H20N2O6S |
| Solubility | ≤30mg/ml in DMSO;10mg/ml in dimethyl formamide |
| Chemical Name | 2-[[[4-(1,3-dioxo-1H-benz[de]isoquinolin-2(3H)-yl)butyl]amino]sulfonyl]-benzoic acid |
| SDF | Download SDF |
| Canonical SMILES | OC(c(cccc1)c1S(NCCCCN(C(c1cccc2c1c1ccc2)=O)C1=O)(=O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure