Amino Acids & Peptides
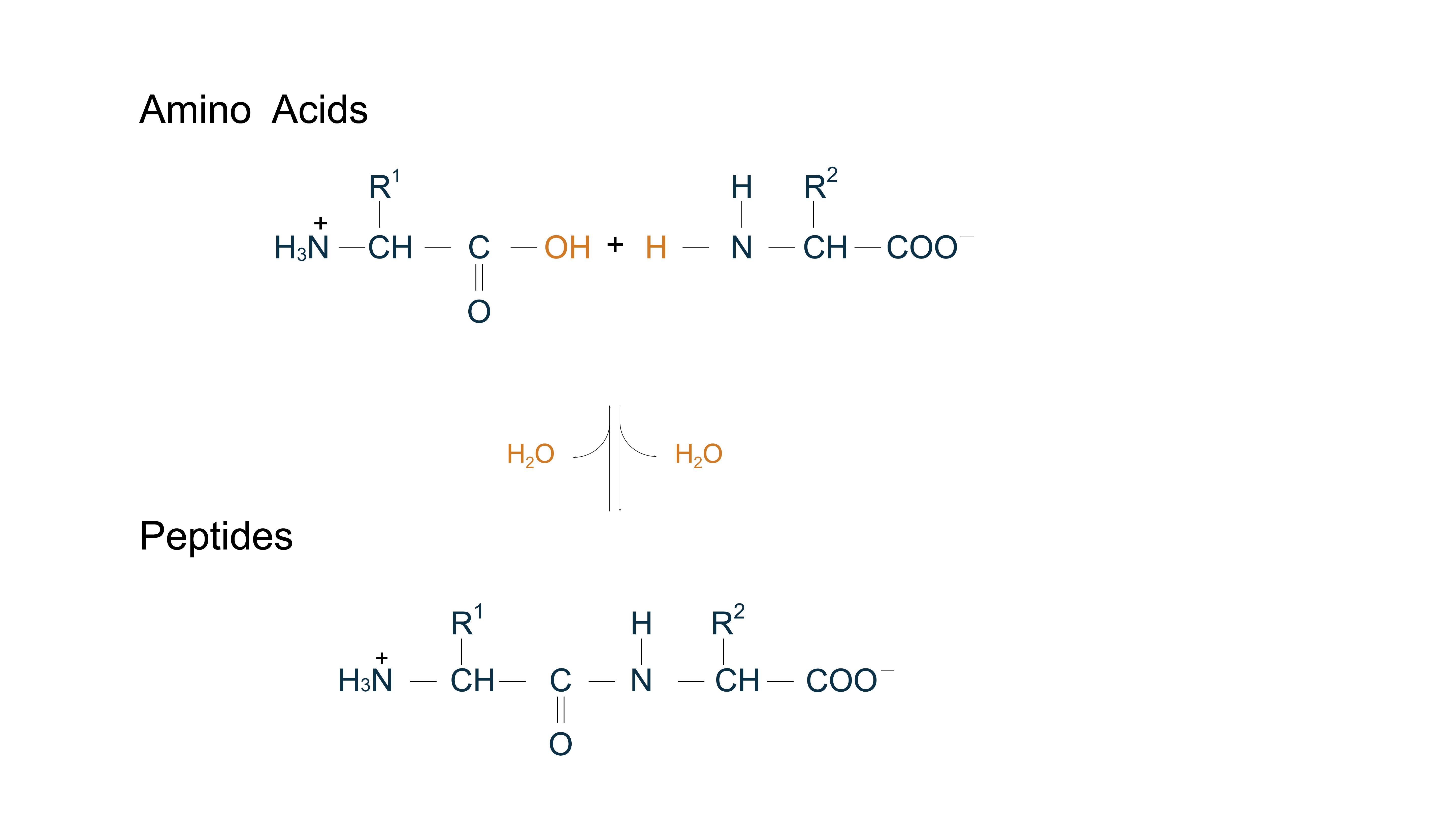

Amino acids are fundamental organic molecules with a common structure: an amino group (–NH₂), a carboxyl group (–COOH), and a side chain (or R group) that differs their properties. 20 α-amino acids are commonly found in proteins, serving as protein building blocks. Some of less common amino acids also have important functions, serving either as constituents of proteins (through modification of common amino acid residues after protein synthesis) or as metabolic intermediates. As amino acids are essential for life, they are used as supplements in cell culture media and as key materials for metabolic studies. Uncommon or modified amino acids are utilized in drug development.
Amino acids can be covalently joined through a peptide bond, formed by dehydration from the α-carboxyl group of one amino acid and the α-amino group of another via condensation reaction, allowing them to polymerize into peptides and proteins. Molecules called peptides generally have molecular weights below 10,000, whereas those called proteins have higher molecular weights. Mainly derived from natural sources or their analogs, peptides exhibit diverse biological activities (antimicrobial, antiviral, antioxidant, immunomodulatory) with high target affinity, stable efficacy, low immunogenicity, and minimal toxicity. Advances in biotechnology and synthesis have expanded their applications in disease research, drug development, and vaccine development. Clinically, peptide-based drugs now address cancer, hepatitis, diabetes, and HIV/AIDS.
-
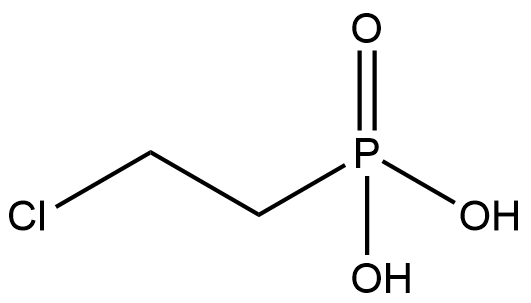 BA9580 EthephonSummary: A plant growth regulator.
BA9580 EthephonSummary: A plant growth regulator. -
 BA9585 GadoteridolSummary: Gadoteridol is a gadolinium-based MRI contrast agent.
BA9585 GadoteridolSummary: Gadoteridol is a gadolinium-based MRI contrast agent. -
 BA9586 3-O-Methyl-D-glucopyranoseSummary: A biochemical reagent.
BA9586 3-O-Methyl-D-glucopyranoseSummary: A biochemical reagent. -
 BA9599 BicineSummary: Bicine is a buffer within the scope of physiological studies.
BA9599 BicineSummary: Bicine is a buffer within the scope of physiological studies. -
 BA9603 CHESSummary: CHES (N-Cyclohexyltaurine) is zwitterionic buffer.
BA9603 CHESSummary: CHES (N-Cyclohexyltaurine) is zwitterionic buffer. -
 BA9604 ThyroglobulinSummary: Thyroglobulin is a 660 kDa dimeric glycoprotein produced by thyroid follicular cells and used exclusively within the thyroid gland.
BA9604 ThyroglobulinSummary: Thyroglobulin is a 660 kDa dimeric glycoprotein produced by thyroid follicular cells and used exclusively within the thyroid gland. -
 BA9605 2-Thio-PAFSummary: 2-Thio-PAF is a synthetic analog of PAF.
BA9605 2-Thio-PAFSummary: 2-Thio-PAF is a synthetic analog of PAF. -
 BA9607 Polyquaternium-1Summary: Polyquaternium-1 (Polidroniumchloride) is a polycationic ophthalmic preservative.
BA9607 Polyquaternium-1Summary: Polyquaternium-1 (Polidroniumchloride) is a polycationic ophthalmic preservative. -
 BA9611 5-Hexen-1-olSummary: 5-Hexen-1-ol, an aromatic substance with an aroma similar to substances such as herbs, tea and citrus.
BA9611 5-Hexen-1-olSummary: 5-Hexen-1-ol, an aromatic substance with an aroma similar to substances such as herbs, tea and citrus. -
 BA9613 NHS-PEG1-SS-PEG1-NHSSummary: NHS-PEG1-SS-PEG1-NHS is a reversible linker of biomolecules interacting with active small molecules.
BA9613 NHS-PEG1-SS-PEG1-NHSSummary: NHS-PEG1-SS-PEG1-NHS is a reversible linker of biomolecules interacting with active small molecules.


