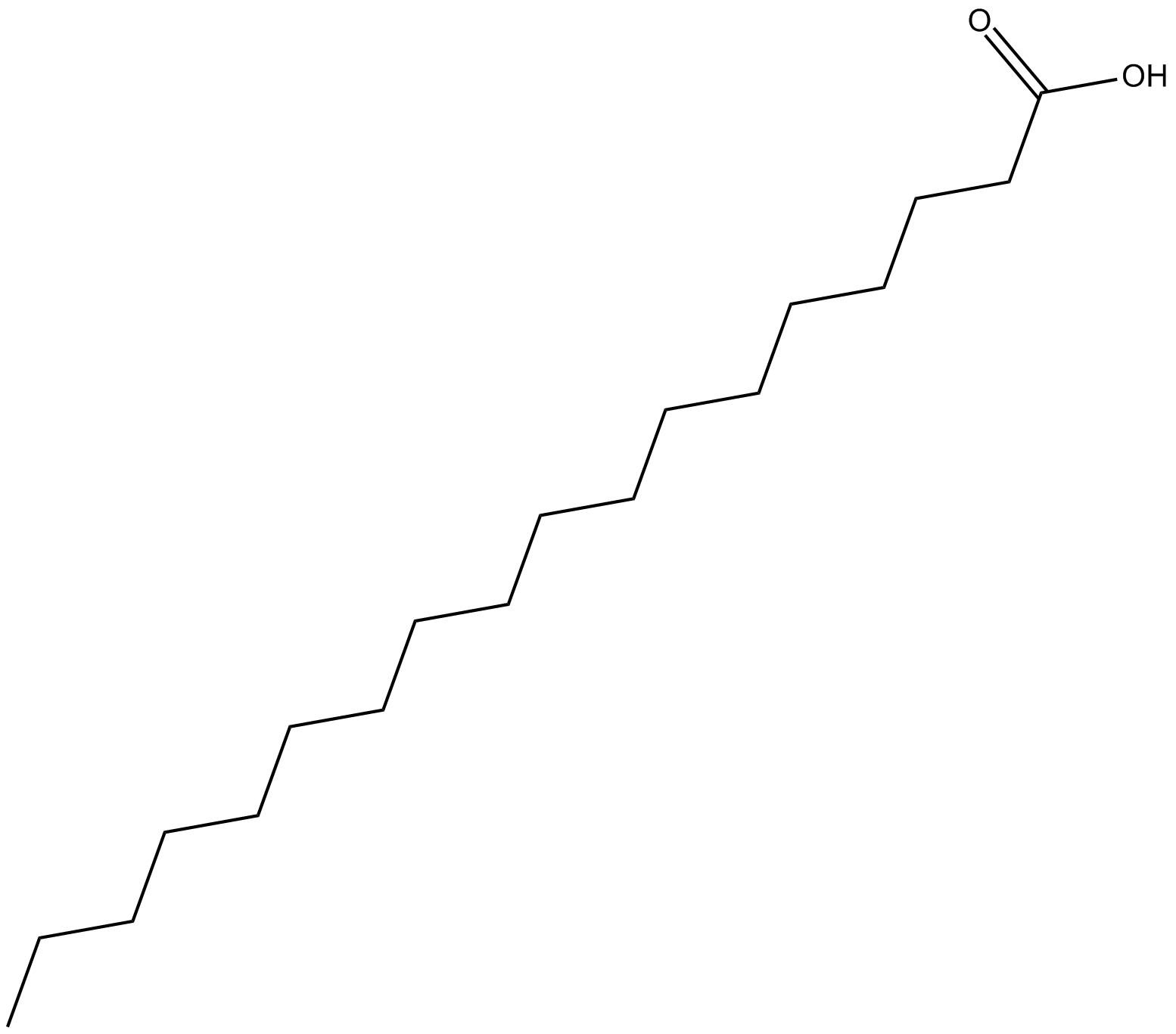
9,12-Octadecadiynoic Acid
Ki: 0.6 μM for ram seminal vesicle COX
9,12-Octadecadiynoic acid is a COX and lipoxygenase inhibitor.
Cyclooxygenase (COX) is an enzyme responsible for the formation of prostanoids, such as thromboxane and prostaglandins. Pharmaceutical inhibition of COX can lead to relief from the symptoms of inflammation and pain. Nonsteroidal anti-inflammatory drugs, including aspirin and ibuprofen, display their effects via inhibition of COX.
In vitro: 9,12-Octadecadiynoic acid was identified as an inhibitor of both COX and lipoxygenase, which could inhibit ram seminal vesicle COX. 9,12-Octadecadiynoic acid was found to be a more effective inhibitor of COX-1 than of 15-LO when used at a concentration of 48 μM [1,2]. In another study, fatty acids from natural sources including 9,12-octadecadiynoic acid were isolated and assayed for their effect on the bioconversion of arachidonic acid into prostaglandin E2. Two very active cyclooxygenase inhibitors were discovered, namely jacarandic acid and the synthetic 8Z, 10E, 12E-octadecatrienoic acid. Earlier described potent inhibitors showed the following IC50 values: 1.3 μM for indomethacin; 1.3 μM for 9,12-octadecadiynoic acid, 2.7 μM for 8Z, 12E, 14Z-eicosatrienoic acid; 4.4 μM for 5,8,11,14-eicosatetraynoic acid [3].
In vivo: Up to now, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Vanderhoek, J. Y. and Lands, W.E.M. Acetylenic inhibitors of sheep vesicular gland oxygenase. Biochimica et Biophysica Acta 296, 374-381 (1973).
[2] Downing, D. T.,Barve, J.A.,Gunstone, F.D., et al. Structural requirements of acetylenic fatty acids for inhibition of soybean lipoxygenase and prostaglandin synthase. Biochimica et Biophysica Acta 280, 343-347 (1972).
[3] Nugteren DH, Christ-Hazelhof E. Naturally occurring conjugated octadecatrienoic acids are strong inhibitors of prostaglandin biosynthesis. Prostaglandins. 1987 Mar;33(3):403-17.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 276.4 |
| Cas No. | 2012-14-8 |
| Formula | C18H28O2 |
| Synonyms | Ro 3-1314 |
| Solubility | ≤10mg/ml in ethanol;10mg/ml in DMSO;10mg/ml in methanol;10mg/ml in acetone;10mg/ml in acetonitrile |
| Chemical Name | 9,12-octadecadiynoic acid |
| SDF | Download SDF |
| Canonical SMILES | CCCCCCCCCCCCCCCCCC(O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
-
Purity = 98.00%
- COA (Certificate Of Analysis)
- MSDS (Material Safety Data Sheet)
- Datasheet
Chemical structure