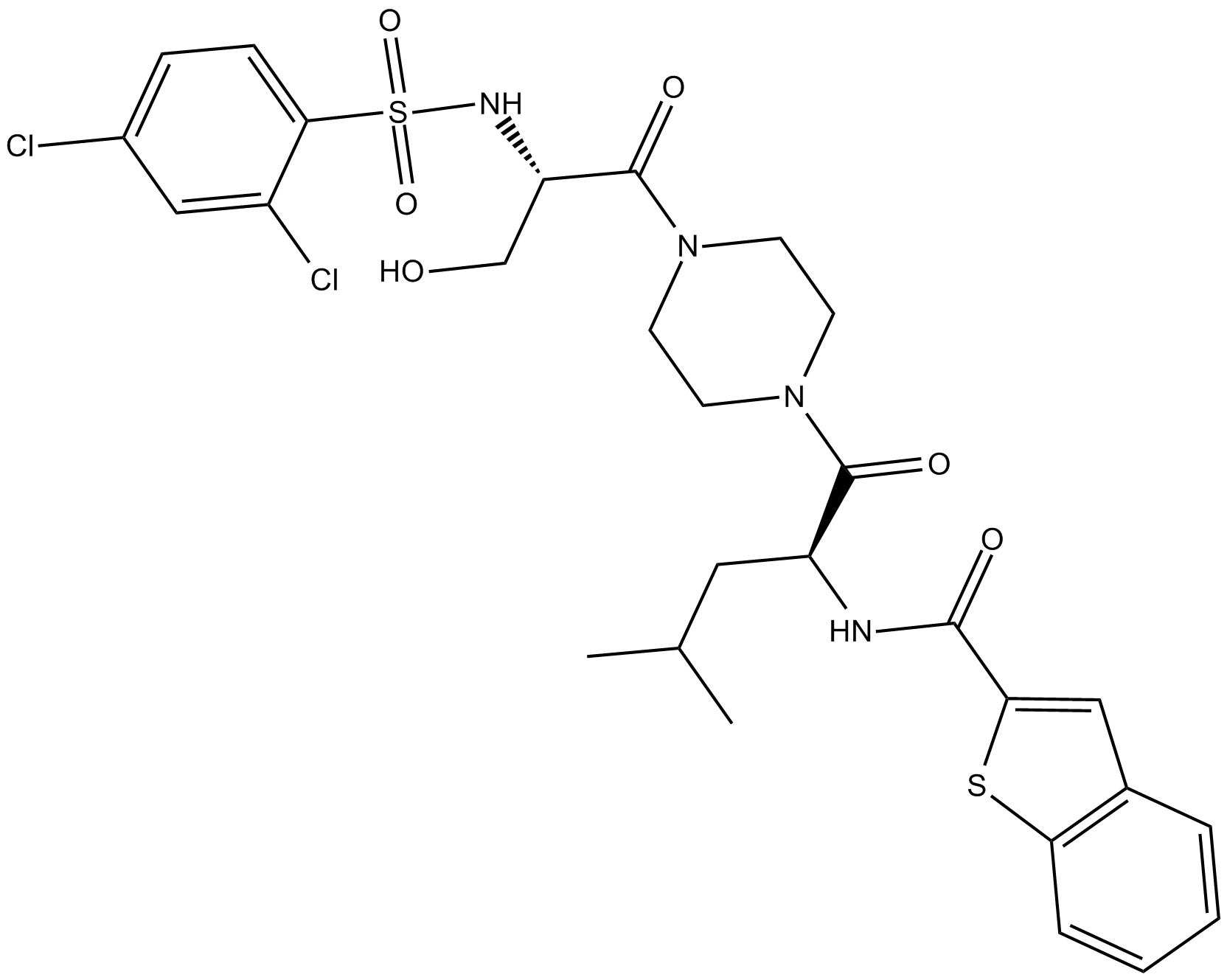
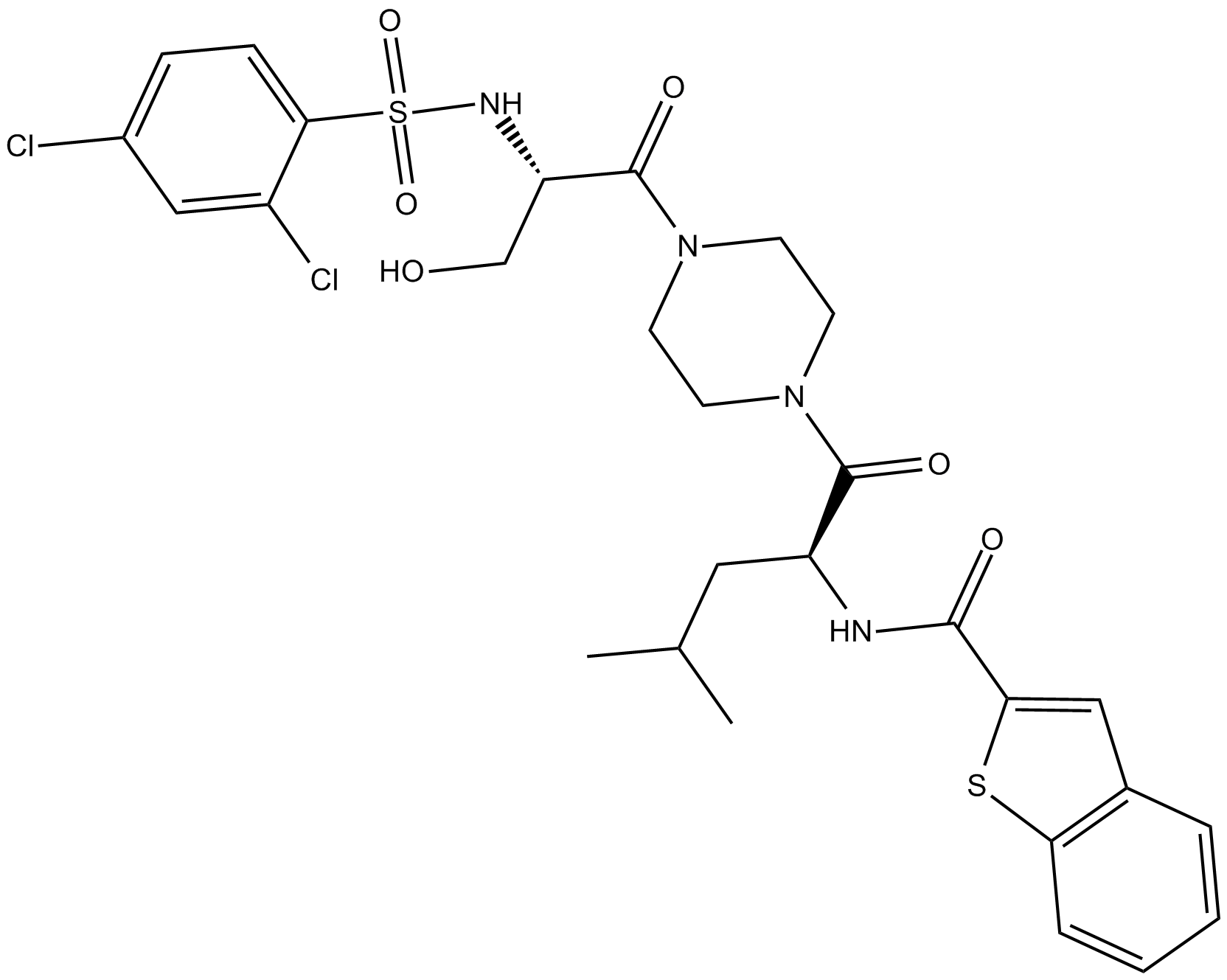
GSK1016790A
GSK1016790A is a small molecule TRPV4 channel activator [1].
TRPV4 is a Ca2+-permeable, nonselective cation channel that has been involved in multiple physiologic functions, such as the regulation of systemic osmotic pressure, vascular function, skin barrier function, airway- and lung function, and in pain. The channel is activated by osmotic, mechanical, chemical cues and thermal changes. Channel activation can be sensitized by inflammation and injury. Mutations of TRPV4 may lead to premature osteoarthritis, skeletal dysplasias, and neurological motor function disorders [2].
In mouse and human TRPV4-expressing human embryonic kidney (HEK) cells, GSK1016790A elicited Ca2+ influx with the EC50 values of 18 and 2.1 nM, respectively. GSK1016790A dose-dependently evoked the activation of TRPV4 whole-cell currents at concentrations above 1 nM [1]. TRPV4 activation with GSK1016790A contracted TRPV4+/+ mouse bladders in vitro. Infusion of GSK1016790A into the bladders of TRPV4+/+ mice induced bladder overactivity with no effect in TRPV4-/- mice [1].
References:
[1] Thorneloe K S, Sulpizio A C, Lin Z, et al. N-((1S)-1-{[4-((2S)-2-{[(2, 4-dichlorophenyl) sulfonyl] amino}-3-hydroxypropanoyl)-1-piperazinyl] carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I[J]. Journal of Pharmacology and Experimental Therapeutics, 2008, 326(2): 432-442.
[2] Nilius B, Owsianik G, Voets T, et al. Transient receptor potential cation channels in disease[J]. Physiological reviews, 2007, 87(1): 165-217.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 655.6 |
| Cas No. | 942206-85-1 |
| Formula | C28H32Cl2N4O6S2 |
| Solubility | ≥50.4 mg/mL in DMSO; ≥26.3 mg/mL in EtOH; insoluble in H2O |
| Chemical Name | N-[(1S)-1-[[4-[(2S)-2-[[(2,4-dichlorophenyl)sulfonyl]amino]-3-hydroxy-1-oxopropyl]-1-piperazinyl]carbonyl]-3-methylbutyl]-benzo[b]thiophene-2-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CC=C(S(N[C@H](C(N2CCN(C([C@@H](NC(C3=CC4=C(C=CC=C4)S3)=O)CC(C)C)=O)CC2)=O)CO)(=O)=O)C(Cl)=C1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Quality Control & MSDS
- View current batch:
Chemical structure