E 64d
E-64d (CAS No. 88321-09-9) is a membrane-permeable cysteine protease inhibitor derived from E-64c, acting via covalent modification of the proteolytic active site thiol group. It demonstrates inhibitory activity primarily against calpain, a calcium-dependent cysteine protease involved in diverse cellular processes. E-64d readily penetrates intact cells, allowing researchers to inhibit intracellular protease activity without disruption. In intact platelet models, short incubation with E-64d results in persistent suppression of calpain activity even after removal of extracellular inhibitor by washing. Experimentally, it is commonly utilized to investigate calpain's role in physiological and pathological events, particularly in platelets and cellular apoptosis pathways. The typical reported IC50 value of E-64d against calpain is approximately 0.5–1 μM.
References:
1. M. Tamai, K. Matsumoto, S. Omura, I. Koyama, Y. Ozawa, K. Hanada J. Pharmacobio-Dyn., 9 (1986), pp. 672–677
2. E. B. McGowan, E. Becker, and T. C. Detwiler, INHIBITION OF CALPAIN IN INTACT PLATELETS BY THE THIOL PROTEASE INHIBITOR E-64d. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS , Vol. 158, No. 2, 1989
3. Carmen JC, Sinai AP. The Differential Effect of Toxoplasma Gondii Infection on the Stability of BCL2-Family Members Involves Multiple Activities. Front Microbiol. 2011 Jan 24;2:1.
- 1. Lu Jiang, Shilong Zhang, et al. "Photoexcited CRY1 physically interacts with ATG8 to regulate selective autophagy of HY5 and photomorphogenesis in Arabidopsis." Plant Cell. 2025 Aug 8:koaf196. PMID: 40795357
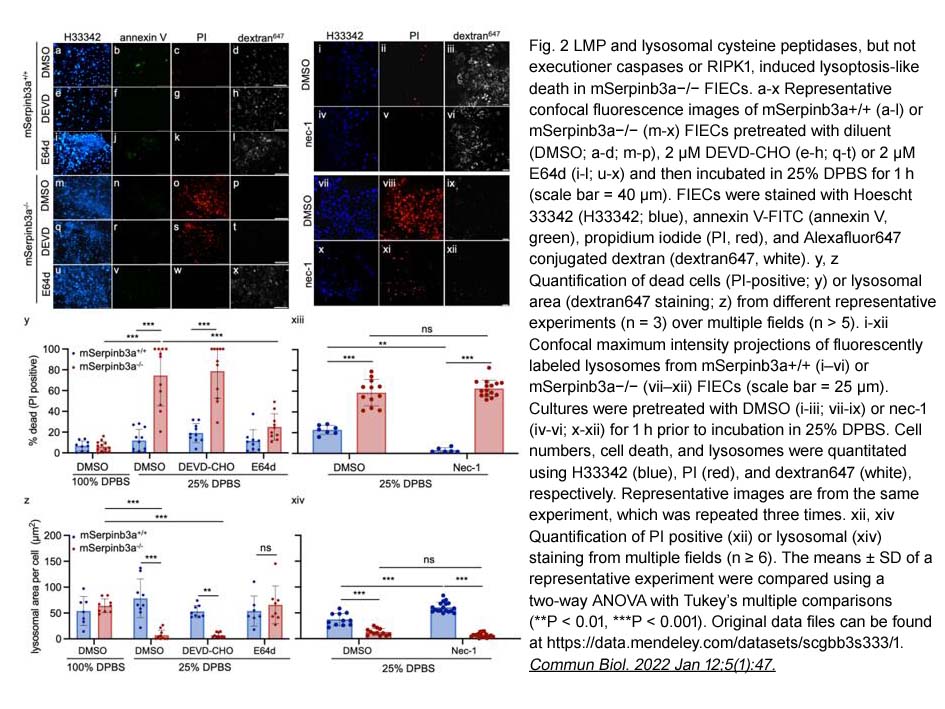
- 2. Cliff J. Luke, Stephanie Markovina, et al. "Lysoptosis is an evolutionarily conserved cell death pathway moderated by intracellular serpins." Commun Biol. 2022 Jan 12;5(1):47. PMID:35022507
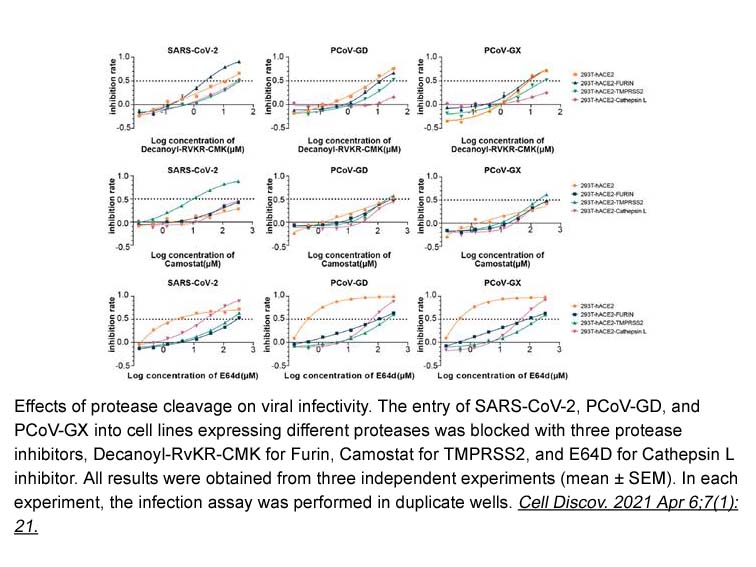
- 3. Jianhui Nie, Qianqian Li, et al. "Functional comparison of SARS-CoV-2 with closely related pangolin and bat coronaviruses." Cell Discov. 2021 Apr 6;7(1):21. PMID:33824288
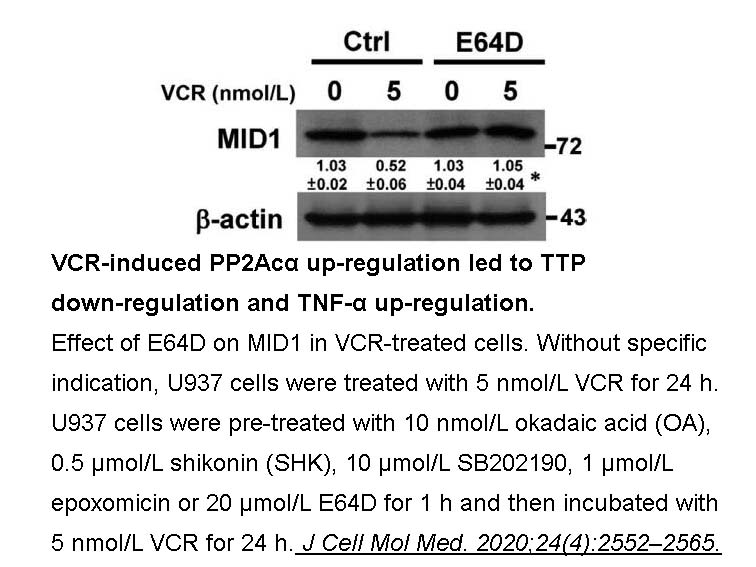
- 4. Wang LJ, Chiou JT, et al. "SIRT3, PP2A and TTP protein stability in the presence of TNF-α on vincristine-induced apoptosis of leukaemia cells." J Cell Mol Med. 2020;24(4):2552–2565. PMID:31930676
- 5. Dziekan, Jerzy Michal. "Molecular analysis of antimalarial therapies : from drug development to mode of action." Nanyang Technological University. 2019.
- 6. Maxine Bauzon, Penelope M. Drake, et al. "Maytansine-bearing antibody-drug conjugates induce in vitro hallmarks of immunogenic cell death selectively in antigen-positive target cells." OncoImmunology.Received 09 Aug 2018, Accepted 12 Dec 2018, Published online: 22 Jan 2019.
- 7. An H, Harper JW. "Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy." Nat Cell Biol. 2017 Dec 11. PMID:29230017
- 8. Tsai YC, Kotiya A, et al. "Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type A intoxication." Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):E5158-E5166. PMID:28584101
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 342.43 |
| Cas No. | 88321-09-9 |
| Formula | C17H30N2O5 |
| Solubility | insoluble in H2O; ≥17.12 mg/mL in DMSO; ≥18.5 mg/mL in EtOH |
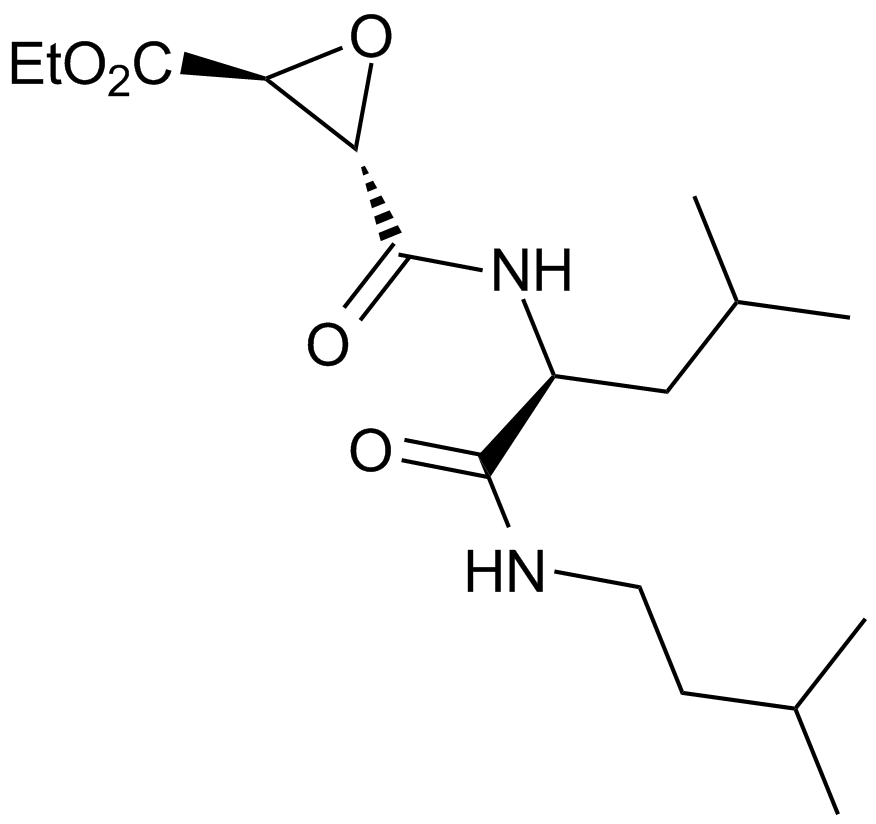
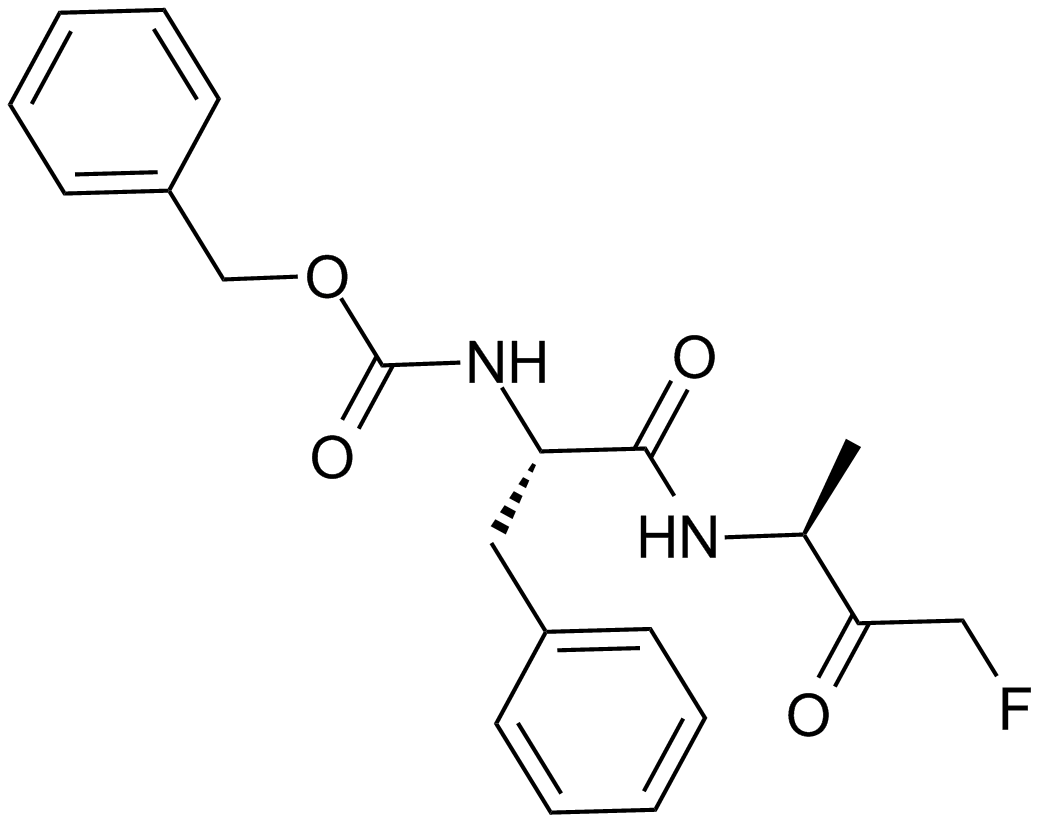
| Chemical Name | ethyl (2S,3S)-3-[[(2S)-4-methyl-1-(3-methylbutylamino)-1-oxopentan-2-yl]carbamoyl]oxirane-2-carboxylate |
| SDF | Download SDF |
| Canonical SMILES | CCOC([C@H]1O[C@@H]1C(N[C@@H](CC(C)C)C(NCCC(C)C)=O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment: [1] | |
|
Cell lines |
Platelets |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
50 μg/ml, 10 min |
|
Applications |
Platelets were activated with A23187 plus calcium, a condition known to lead to calpain-catalyzed proteolysis of ABP and talin. In the absence of inhibitor, A23187 led to complete degradation of ABP and talin. E 64d, the permeant inhibitor, did inhibit intracellular proteolysis. Some inhibition was observed at the lowest concentration tested, 20 μg/ml, and essentially complete inhibition was obtained with 50 μg/ml. |
| Animal experiment: [2] | |
|
Animal models |
Male Sprague–Dawley rats |
|
Dosage form |
Intraperitoneal injection, 4 μg |
|
Applications |
After E-64d treatment, mice were treated with penicillin to induce recurrent seizures. The result showed that E-64d remarkably reduced the aberrant mossy fiber sprouting in the supragranular region of dentate gyrus and CA3 subfield of hippocampus. In rats without E-64d treatment, there was prominent aggregation of mossy fiber terminals in the dentate gyrus and in the stratum pyramidale of CA3 subfield. In rats treated with E-64d, the aggregation of mossy fiber terminals was remarkably decreased in both the supragranular region of dentate gyrus and CA3 subfield. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] McGowan E B, Becker E, Detwiler T C. Inhibition of calpain in intact platelets by the thiol protease inhibitor E-64d. Biochemical and biophysical research communications, 1989, 158(2): 432-435. [2] Ni H, Ren S, Zhang L, et al. Expression profiles of hippocampal regenerative sprouting-related genes and their regulation by E-64d in a developmental rat model of penicillin-induced recurrent epilepticus. Toxicology letters, 2013, 217(2): 162-169. |
|
| E-64d, a synthetic analog of E-64 and ethyl ester of E-64c, is an irreversible, membrane-permeable inhibitor of lysosomal and cytosolic cysteine proteases. E-64d inhibits calpain and the cysteine proteases cathepsins F, K, B, H, and L. | ||||||
| Targets | cathepsins F | cathepsins K | cathepsins B | cathepsins H | cathepsins L | |
| IC50 | ||||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
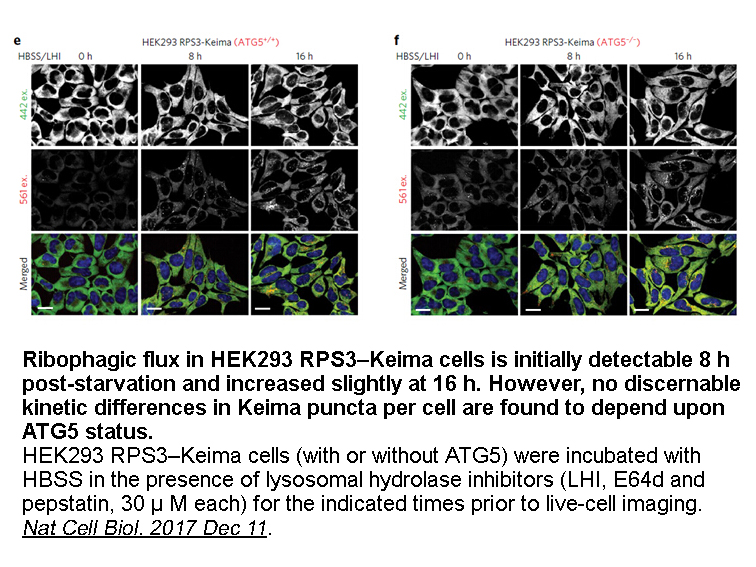
Related Biological Data
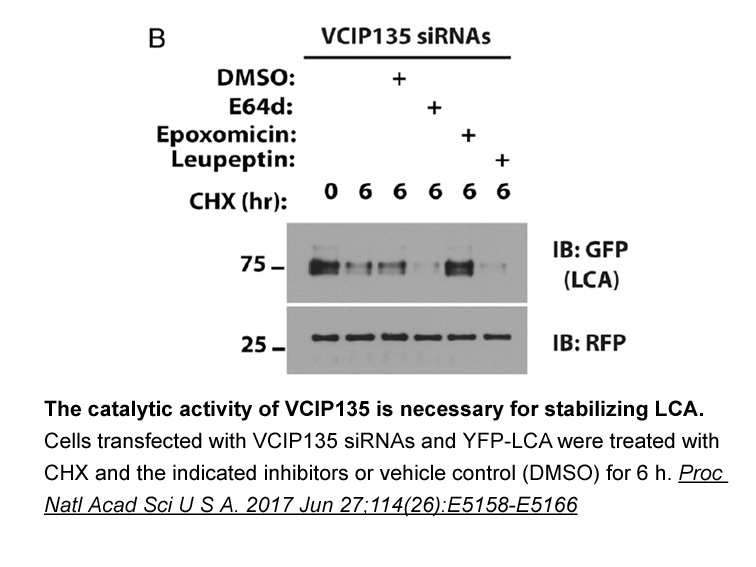
Related Biological Data
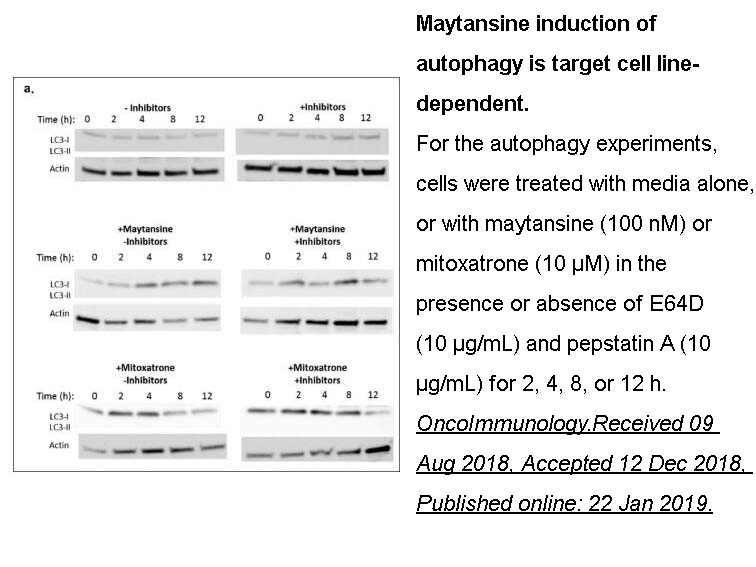
Related Biological Data
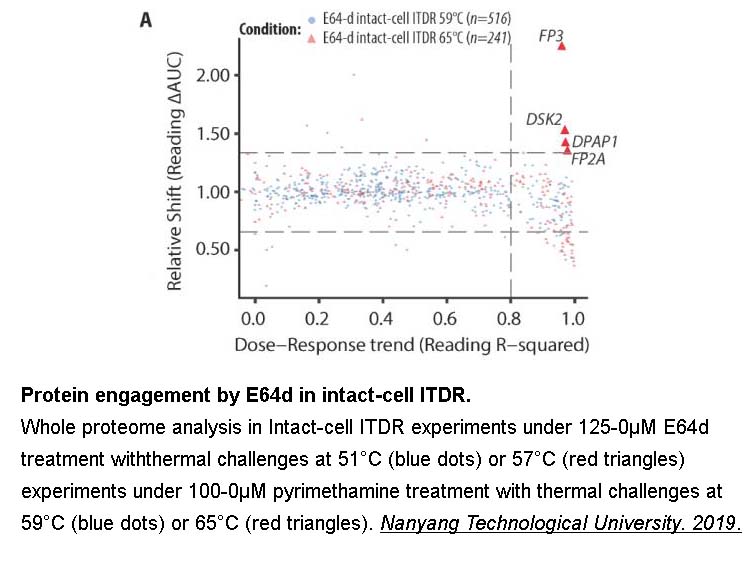
Related Biological Data