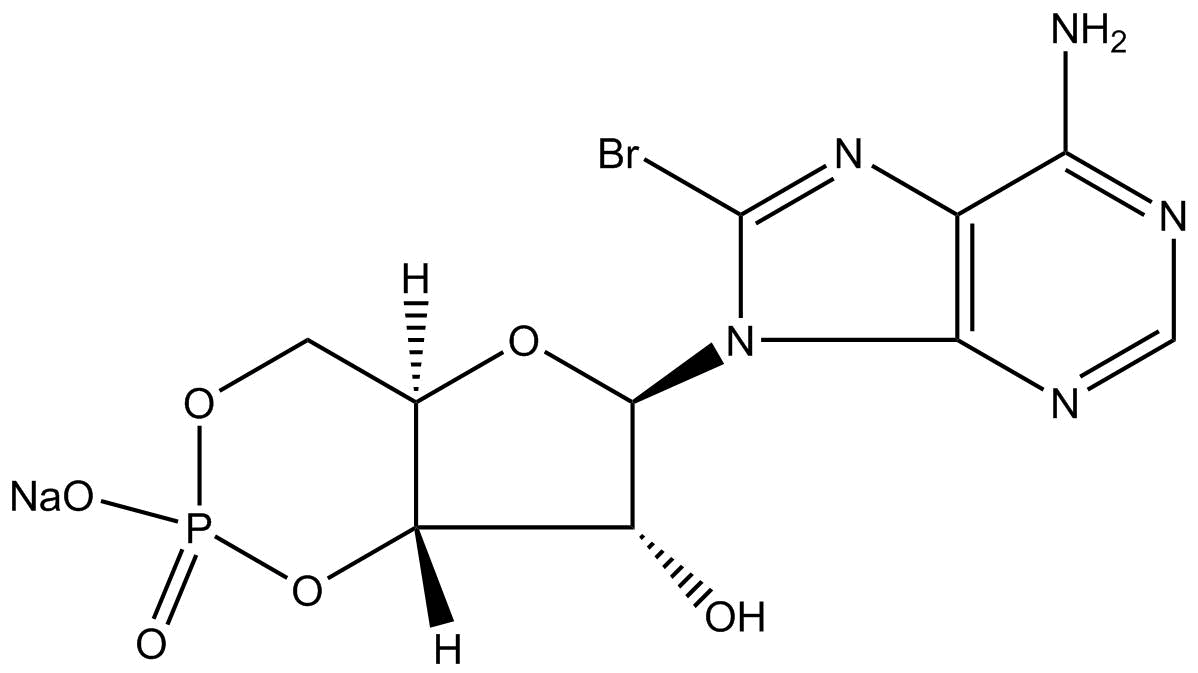
8-Bromo-cAMP, sodium salt
8-Bromo-cAMP is a cAMP-analogues. [1]
cAMP can have an effect on the proliferative response of a cell. Either the effect is a positive or a negative regulator depending on the cell types. There is also variable effect of cAMP in hematopoietic cell lines. CAMP-analogues can significantly inhibit the erythropoietin-stimulated proliferation.[1]
8-Bromo-cAMP increased the cellular content of mRNA encoding the hCG a- and β-subunits and prevented the increase in fibronectin mRNA. This is determined by blot hybridization analysis using specific cDNA probes. 8-Bromo-cAMP also induced phosphorylation of Erk1,2 in AML193 cells. 8-Bromo-cAMP is an agent in AML193 cells and activates Erk1,2 , this condition happens does not accompanied by the involvement of Shc phosphorylation.[1,2]
8-bromo-cAMP increases catecholamine biosynthesis and produces an increase both in the activity and phosphorylation of tyrosine hydroxylase. 8-bromo-cAMP also promotes alterations in the synthesis and secretion of specific proteins, including fibronectin and the subunits of hCG by regulating mRNA expression.[2,3]
References:
[1]Ulloa-Aguirre A,?August AM,?etal. , 8-Bromo-adenosine?3',5'-monophosphate?regulates?expression?of?chorionic?gonadotropin?and fibronectin in human cytotrophoblasts. J Clin Endocrinol Metab.?1987 May;64(5):1002-9.
[2] Rene e M. Y. Barge, J.H. Frederik Falkenburg, etal., 8-Bromo-cAMP induces a proliferative response in an IL-3 dependent leukemic cell line and activates Erk1,2 via a Shc-independent pathway. Biochimica et Biophysica Acta 1355 (1997) 141–146.
[3] Haycock JW, Bennett WF, etal. , Multiple site phosphorylation of tyrosine hydroxylase. Differential regulation in situ by a 8-bromo-cAMP and acetylcholine. J Biol Chem. 1982 Nov 25;257(22):13699-703.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 430.09 |
| Cas No. | 76939-46-3 |
| Formula | C10H10BrN5NaO6P |
| Solubility | ≥43 mg/mL in H2O; insoluble in EtOH; ≥22.43 mg/mL in DMSO with gentle warming and ultrasonic |
| Chemical Name | sodium (4aR,6R,7R,7aS)-6-(6-amino-8-bromo-9H-purin-9-yl)-7-hydroxytetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-2-olate 2-oxide |
| SDF | Download SDF |
| Canonical SMILES | Nc1ncnc([n]2[C@@H]([C@@H]3O)O[C@H](CO4)[C@H]3OP4([O-])=O)c1nc2Br.[Na+] |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Growth-arrested AML193 cells |
|
Preparation method |
The solubility of this compound in sterile water is 100 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months. |
|
Reacting condition |
0 ~ 15 μM |
|
Applications |
In growth-arrested AML193 cells, 8-Bromo-cAMP significantly increased DNA synthesis, with the ED50 value of approximately 10 μM. At the doses over 2.5 μM, a toxic effect was observed. Moreover, the addition of 1 μM 8-Bromo-cAMP synergized the IL-3-induced mitogenic response. In addition, 8-Bromo-cAMP at the concentration of 1μM resulted in maximal Erk phosphorylation. But the effect of 8-Bromo-cAMP on Erk phosphorylation was transient, with the maximal stimulation shown between 1 and 5 mins. |
|
References: [1]. Rene e M. Y. Barge, J.H. Frederik Falkenburg, etal., 8-Bromo-cAMP induces a proliferative response in an IL-3 dependent leukemic cell line and activates Erk1,2 via a Shc-independent pathway. Biochimica et Biophysica Acta 1355 (1997) 141–146. | |
Quality Control & MSDS
- View current batch:
Chemical structure