CX-4945 (Silmitasertib)
CX-4945 (Silmitasertib) is a potent and selective casein kinase 2 (CK2) inhibitor with IC50 value of 1 nM. It is ATP-competitive and can be taken orally [1].
CX-4945 has been reported to have antiproliferative activity against a wide range of tumor cell lines. It is suggested that CX-4945 suppresses the CK2 regulated PI3K/Akt signaling pathway by inhibiting Akt phosphorylation at Serine 129, but not by activating PTEN. Additionally, cells treated with CX-4945 had a reduction of p21 phophorylation and an up-regulations of total p21 and p27. CX-4945 has been shown to induce cell-cycle arrest at G2/M phase in breast cancer cell line BT-474. It also causes cell-cycle arrest at G1 phase the breast cancer cell line BxPC-3) [1].
In CX-4945 and BxPC-3 derived mouse xenograft model, CX-4945 induced a reduction of phos-p21 expression along with anti-carcinoma effects [1]
References:
[1] Siddiqui-Jain A1, Drygin D, Streiner N, Chua P, Pierre F, O'Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG,Anderes K. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010 Dec 15; 70 (24): 10288-98.
- 1. Andrew Dimond, Do Hyeon Gim, et al. "PBK/TOPK mediates Ikaros, Aiolos and CTCF displacement from mitotic chromosomes and alters chromatin accessibility at selected C2H2-zinc finger protein binding sites." bioRxiv. April 23, 2024.
- 2. Niechi I, Erices JI, et al. "Cancer Stem Cell and Aggressiveness Traits Are Promoted by Stable Endothelin-Converting Enzyme-1c in Glioblastoma Cells." Cells 2023 Feb 03;12(3) PMID: 36766848
- 3. Prabhakar AT, James CD, et al. "Human papillomavirus 16 E2 interaction with TopBP1 is required for E2 and viral genome stability during the viral life cycle." bioRxiv 2023 Jan 13; PMID: 36712128
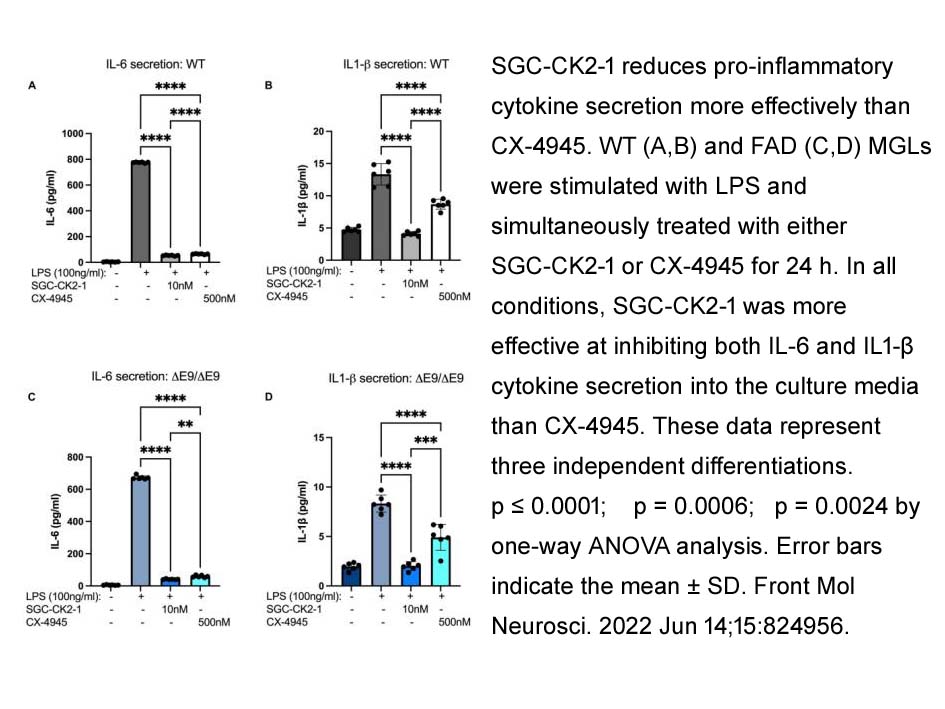
- 4. Swati Mishra, Chizuru Kinoshita, et al. "Evaluation of a Selective Chemical Probe Validates That CK2 Mediates Neuroinflammation in a Human Induced Pluripotent Stem Cell-Derived Mircroglial Model." Front Mol Neurosci. 2022 Jun 14;15:824956. PMID: 35774866
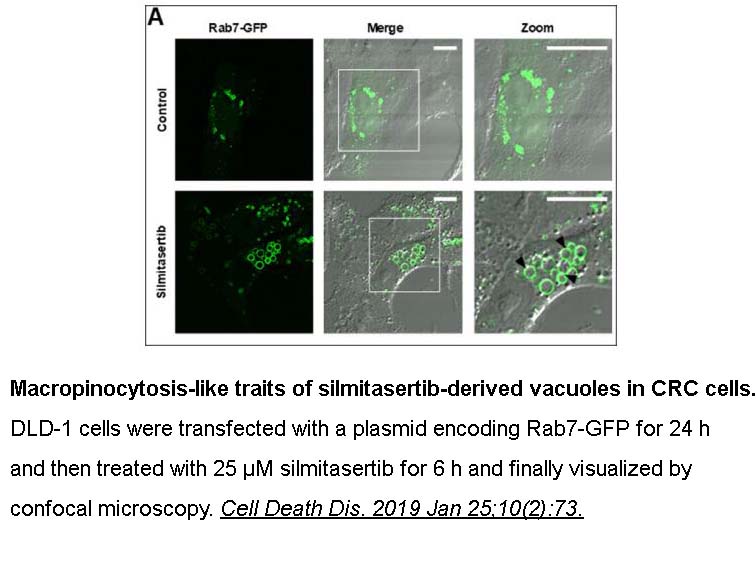
- 5. Silva-Pavez E, Villar P, et al. "CK2 inhibition with silmitasertib promotes methuosis-like cell death associated to catastrophic massive vacuolization of colorectal cancer cells." Cell Death Dis.2019 Jan 25;10(2):73. PMID: 30683840
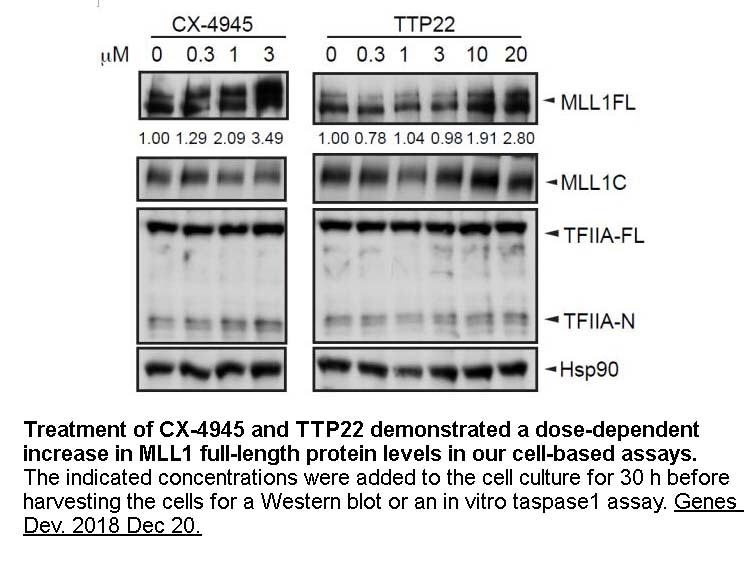
- 6. Zhao Z, Wang L, et al."Regulation of MLL/COMPASS stability through its proteolytic cleavage by taspase1 as a possible approach for clinical therapy of leukemia." Genes Dev. 2019 Jan 1;33(1-2):61-74. PMID: 30573454
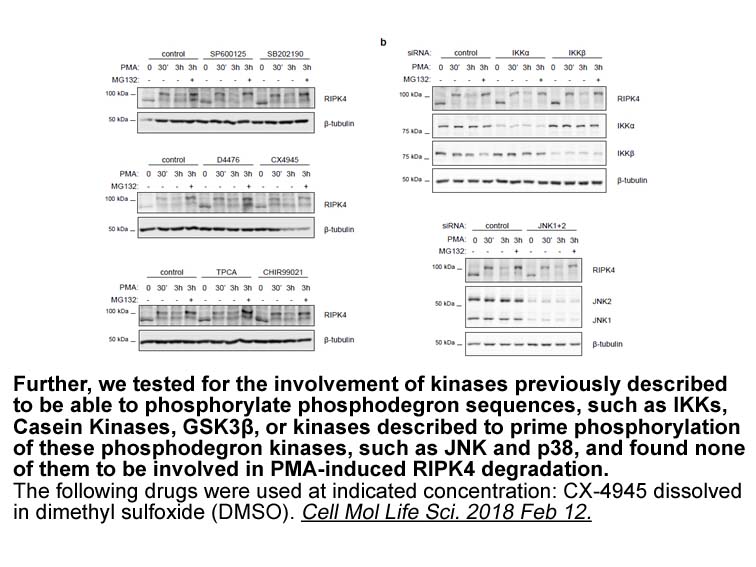
- 7. Tanghe G, Urwyler-Rösselet C, et al. "RIPK4 activity in keratinocytes is controlled by the SCF(β-TrCP) ubiquitin ligase to maintain cortical actin organization." Cell Mol Life Sci. 2018 Feb 12. PMID: 29435596
- 8. Wu F, Qiu J, et al. "Apelin-13 attenuates ER stress-mediated neuronal apoptosis by activating Gα(i)/Gα(q)-CK2 signaling in ischemic stroke." Exp Neurol. 2018 Apr;302:136-144. PMID: 29337146
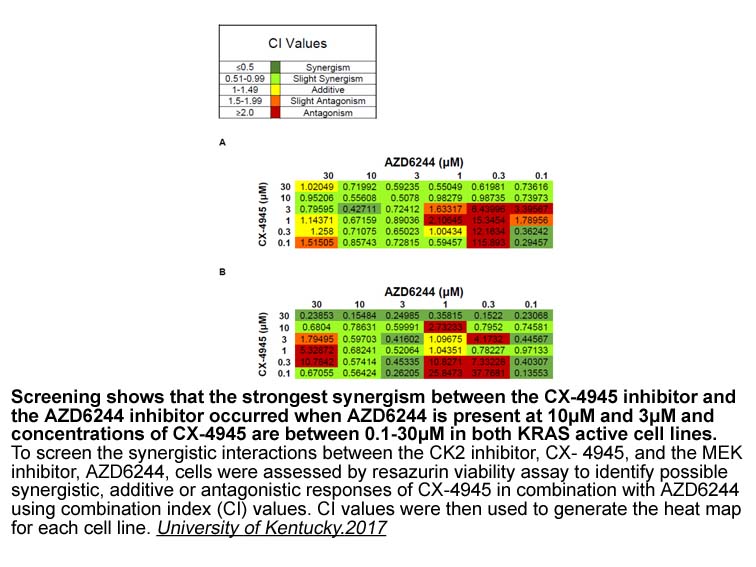
- 9. Krentz Gober, Madeline J. "GENE EXPRESSION PROFILES REVEAL ALTERNATIVE TARGETS OF THERAPEUTICINTERVENTION FOR THE TREATMENT OF DRUG-RESISTANT NON-SMALL CELL LUNG CANCERS" (2017).Thesesand Dissertations--Pharmacy. 78.
- 10. Korb E, Herre M, et al. "Excess Translation of Epigenetic Regulators Contributes to Fragile X Syndrome and Is Alleviated by Brd4 Inhibition." Cell. 2017 Aug 12. PMID: 28823556
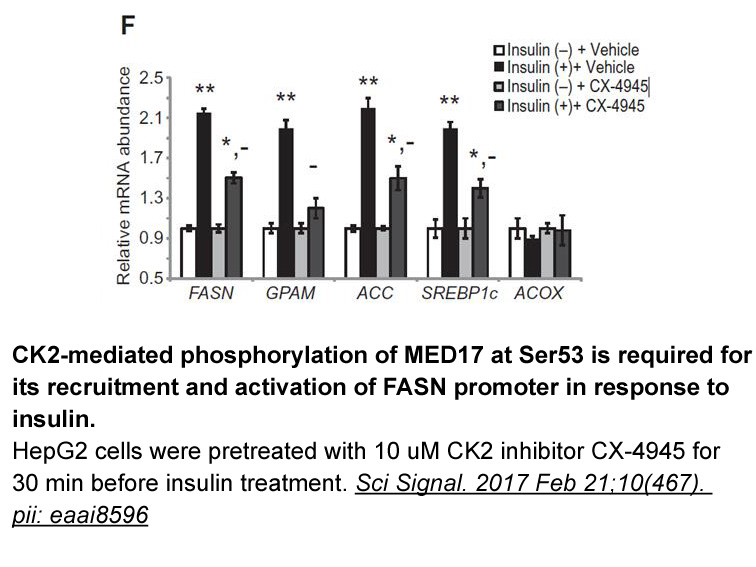
- 11. Viscarra JA, Wang Y, et al. "Transcriptional activation of lipogenesis by insulin requires phosphorylation of MED17 by CK2." Sci Signal. 2017 Feb 21;10(467). pii: eaai8596. PMID: 28223413
- 12. Kubiński K, Masłyk M, Orzeszko A. "Benzimidazole inhibitors of protein kinase CK2 potently inhibit the activity of atypical protein kinase Rio1." Mol Cell Biochem. 2017 Feb;426(1-2):195-203. PMID: 27909846
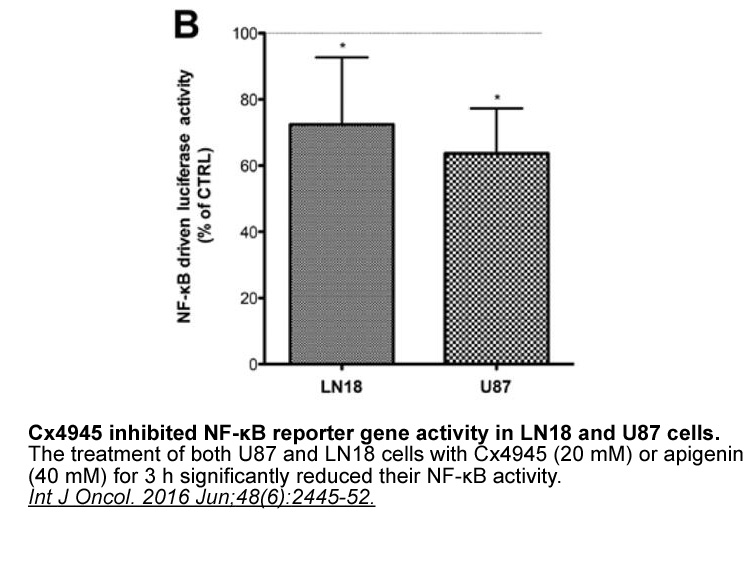
- 13. Dubois N, Willems M, et al. "Constitutive activation of casein kinase 2 in glioblastomas: Absence of class restriction and broad therapeutic potential." Int J Oncol. 2016 Jun;48(6):2445-52. PMID: 27098015
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 349.77 |
| Cas No. | 1009820-21-6 |
| Formula | C19H12ClN3O2 |
| Synonyms | CX 4945; CX4945 |
| Solubility | ≥103.5 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
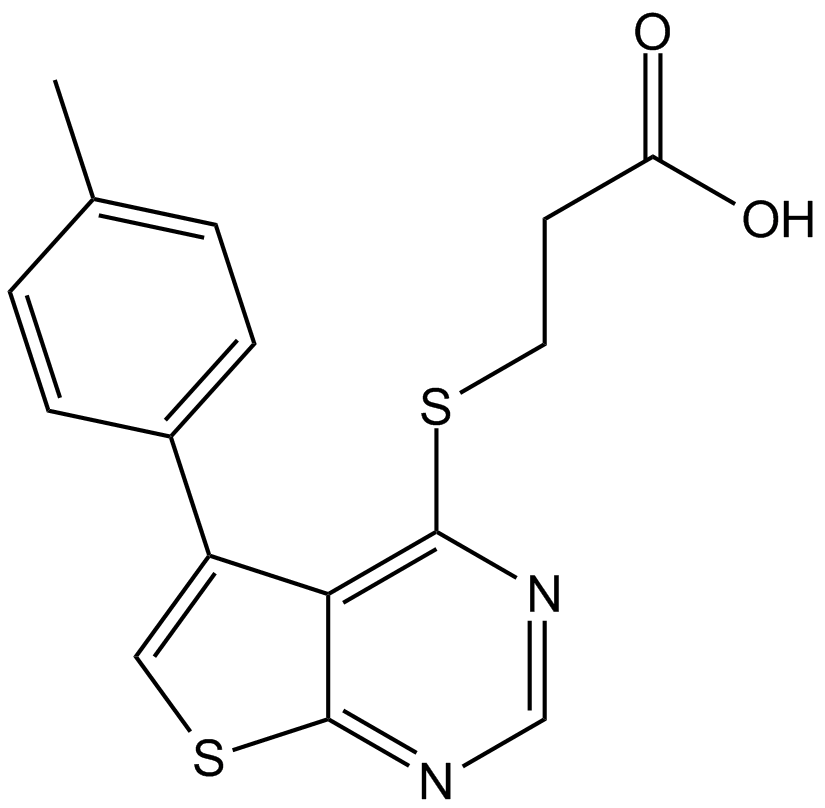
| Chemical Name | 5-(3-chloroanilino)benzo[c][2,6]naphthyridine-8-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | OC(c(cc1)cc2c1c(cncc1)c1c(Nc1cccc(Cl)c1)n2)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
| Cell experiment [1]: | |
|
Cell lines |
Jurkat cells |
|
Preparation method |
The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37°C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
|
Reaction Conditions |
4d; IC50=0.1 μM |
|
Applications |
CK2 inhibition was confirmed by measuring the phosphorylation level of the CK2 specific phosphorylation site on Akt (S129). CX-4945 induced dephosphorylation of Akt (S129) and a rapid dephosphorylation of the Akt substrate p21 (T145). Apoptosis was induced by CX-4945. CX-4945 was also found to potently inhibit endogenous intracellular CK2 activity with an IC50 of 0.1 μM in Jurkat cells. |
| Animal experiment [1]: | |
|
Animal models |
Athymic mice |
|
Dosage form |
75 mg/kg; bid; oral taken |
|
Applications |
CX-4945 was tested for in vivo efficacy in established human prostate PC3 xenograft model in athymic mice. Mice bearing subcutaneous PC3 tumors were treated with CX-4945 (25 mg/kg, 50 mg/kg, and 75 mg/kg, p.o, bid). CX-4945 demonstrated tumor growth inhibition (TGI = 19%, 40%, and 86%, respectively) compared to vehicle treated control, and a dose responsive efficacy was observed. Last, CX-4945 was well tolerated in mice as assessed by minimal changes in body weight during the course of treatment compared to vehicle control. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1] Pierre F, Chua P C, O’Brien S E, et al. Discovery and SAR of 5-(3-chlorophenylamino) benzo [c][2, 6] naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer [J]. Journal of medicinal chemistry, 2010, 54 (2): 635-654. |
|
| Description | CX-4945 (Silmitasertib) is a potent and selective inhibitor of CK2 (casein kinase 2) with IC50 of 1 nM. | |||||
| Targets | CK2α | CK2α' | ||||
| IC50 | 1 nM | 1 nM | ||||
Quality Control & MSDS
- View current batch:
Chemical structure
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data
Related Biological Data